
Image Credits: eMPR
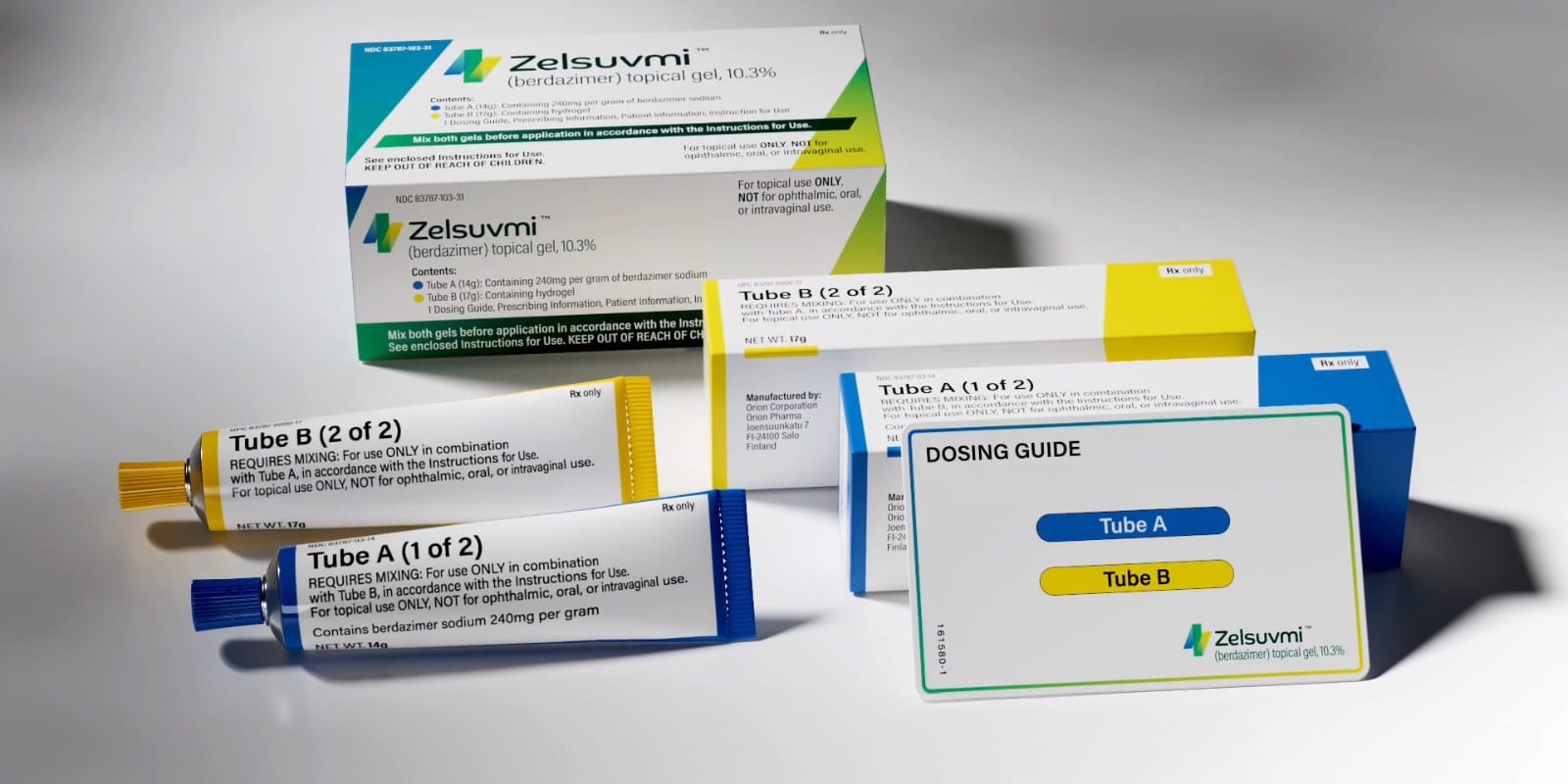
Ligand Pharmaceuticals Incorporated partner Pelthos Therapeutics Inc has commercially introduced ZELSUVMI (berdazimer) topical gel 10.3%, which is an FDA-approved (at-home treatment) for molluscum contagiosum. Ligand has to date earned $5 million in profit payment from Pelthos, adding the introduction of ZELSUVMI. With the completion of the alliance between Channel Therapeutic and Pelthos Therapeutics in July 2025, Ligand now owns up to 56% of Pelthos. The license agreement with Pelthos underlines ligands' entitlement to the 13% royalty on global sales of ZELSUVMI and $5 million in commercial sales profit.
The new launch is a benefit to Ligand and an advancement to Pelthos. Pelthos is stepping forward to accelerate the healthcare market with its new introduction. The continued partnership strategy and now this launch an additional contribution to the healthcare sector. Ligand's partnership with Pelthos shows resilience and efforts. The product’s commercialization in the market offers relief to patients who felt ignored regarding their conditions. The new medication’s effectiveness will further encourage Pelthos' efforts. Despite many skin solution treatments, individuals mainly rely on scientifically proven solutions. The product is an FDA-approved (drug-designated) solution that comes under prescription. Additionally, ZELSUVMI is ruling the healthcare news line. The performance of this medication in the market will double the demand for the product.
ZELSUVMI (berdazimer) topical; gel, 10.3% is a nitric oxide (NO) agent used for the treatment of molluscum contagiosum in pediatric and adult patients, for 1 year and older. ZELSUVMI is a novel drug designated gel, 2024, and the first approved topical medication (prescribed) which is applicable for caregivers at home (or outside the physician's center), patients, parents, or otherwise in different medical environments for the treatment of severe skin infection. The product was initiated with the help of the exclusive nitric oxide-based technology platform ‘NITRICIL™’. Now, Ligand owns NITRICIL™. The safety information and prescription information are at www.zelsuvmi.com.
CEO of Ligand, Todd Davis, said, “Molluscum has affected tons of people in the US, mainly targeting children. We are excited to see ZELSUVMI commercially available in the market as it identifies a major need for the patients and enables at-home treatment for this severe viral skin condition. The profit payment showcases the current progress and the importance of our partnered programs, and also our fueled business model. The business model is built on strategic collaboration, continuous creation for our shareholders, and innovation.”