
Varian, a leading Siemens Healthineers company, completed enrollment and treatment in the FAST-02, a flash radiotherapy clinical trial for the treatment of symptomatic bone metastases in the thorax. The FAST-02 study focuses on painful, extreme bone metastases in the thoracic area and shows a significant step towards introducing this investigational radiotherapy into clinical practice.
The trial was carried out at Cincinnati Children’s Hospital/UC Health proton therapy center and further enrolled 10 participants. The trial aims to evaluate the efficacy of the treatment based on efficacy and side effects of treatment, as assessed by trial participants, considering pain relief. The FAST-02 has stood on the findings from the company’s FAST-01 trial, which analyzed clinical workflow feasibility of treatment-based and flash therapy side effects for bone metastases in the severe participants. The trial was led by the principal investigator, John Perentesis, MD, associate professor of radiation oncology, University of Cincinnati Cancer Center, and MD professor and director of Cancer and Blood Disease Institute, Cincinnati Children’s Hospital, and lead co-investigator Emily Daugherty.
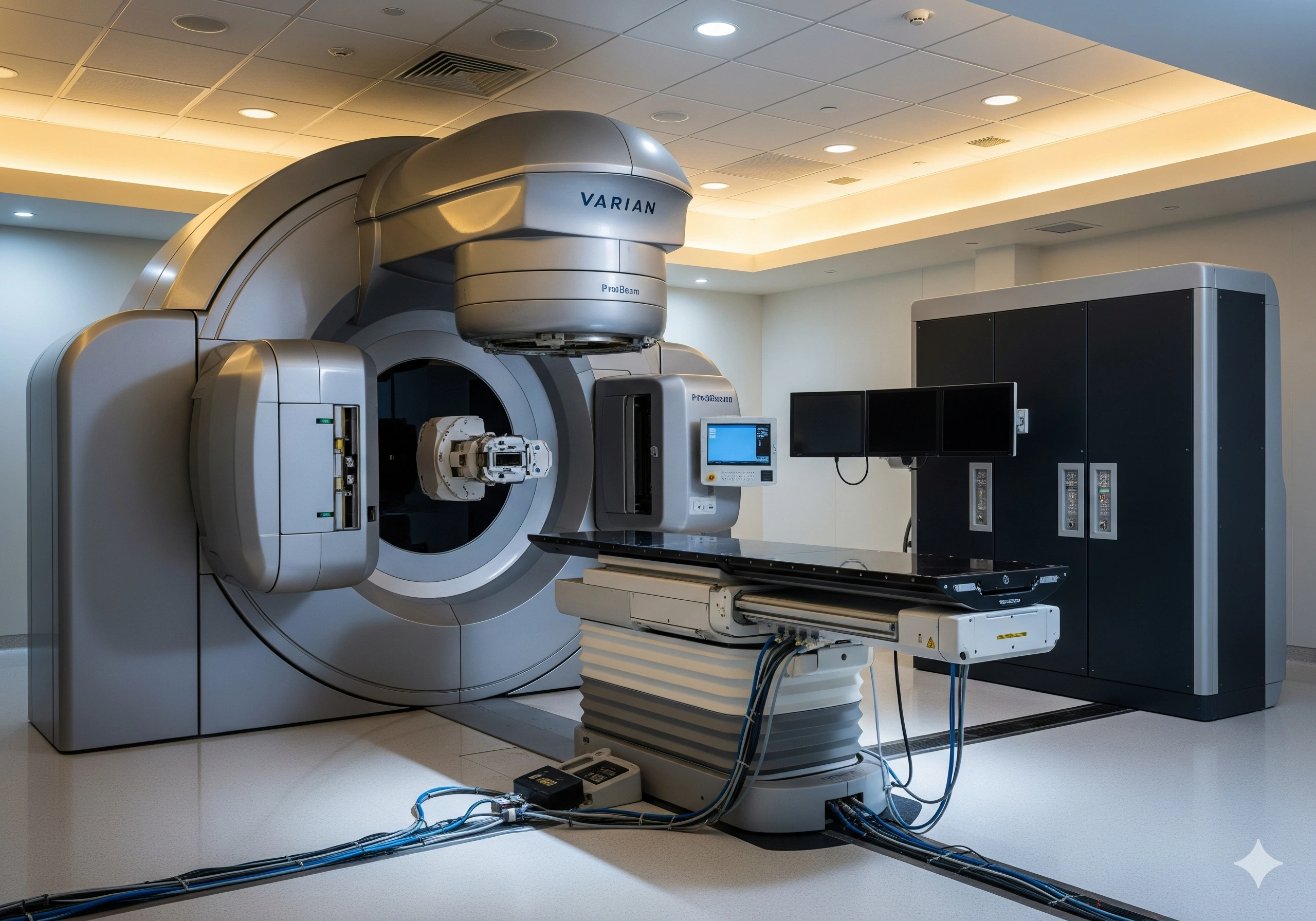
The flash therapy treatment serves at an ultra-high dose rate quickly, in less than one second in over 100 times faster in comparison to conventional radiation therapy. It has elaborated the potency level in the preclinical studies to diminish damage to near healthy tissues while managing effective tumor control. Being a part of the FAST-02 trial, the Varian’s ProBeam proton therapy system was slightly modified to provide an ultra-high dose rate delivery for flash treatments. In different to which, the eclipse treatment planning system was initiated to contribute to the planning for flash therapy. The company is enhancing the flash therapy to bring an integrated system, focusing on quality assurance, treatment delivery, and planning the technologies accordingly.
MD, Professor and director, Cancer and blood disease institute, Cincinnati children’s hospital medical center (CCHMC), John Perentesis, said, “The completion of treatments for FAST-02 is a vital and successive step of our efforts to develop effective and safe flash radiotherapy. This trial has largely supported the groundwork required to shift flash into an advanced clinical setting to discover an innovation that will define radiation oncology best and effectively enhance patient outcomes.”
Executive director and director of medical physics of the CCHMC proton therapy center, Anthony Mascia, said, “The integration of planning and treatment shows a crucial technological achievement.”