February 2026

The global antibodies drug market size was calculated at USD 272.3 billion in 2025, to reach USD 301.71 billion in 2026 is expected to be worth USD 759.36 billion by 2035, expanding at a CAGR of 10.8% from 2026 to 2035.
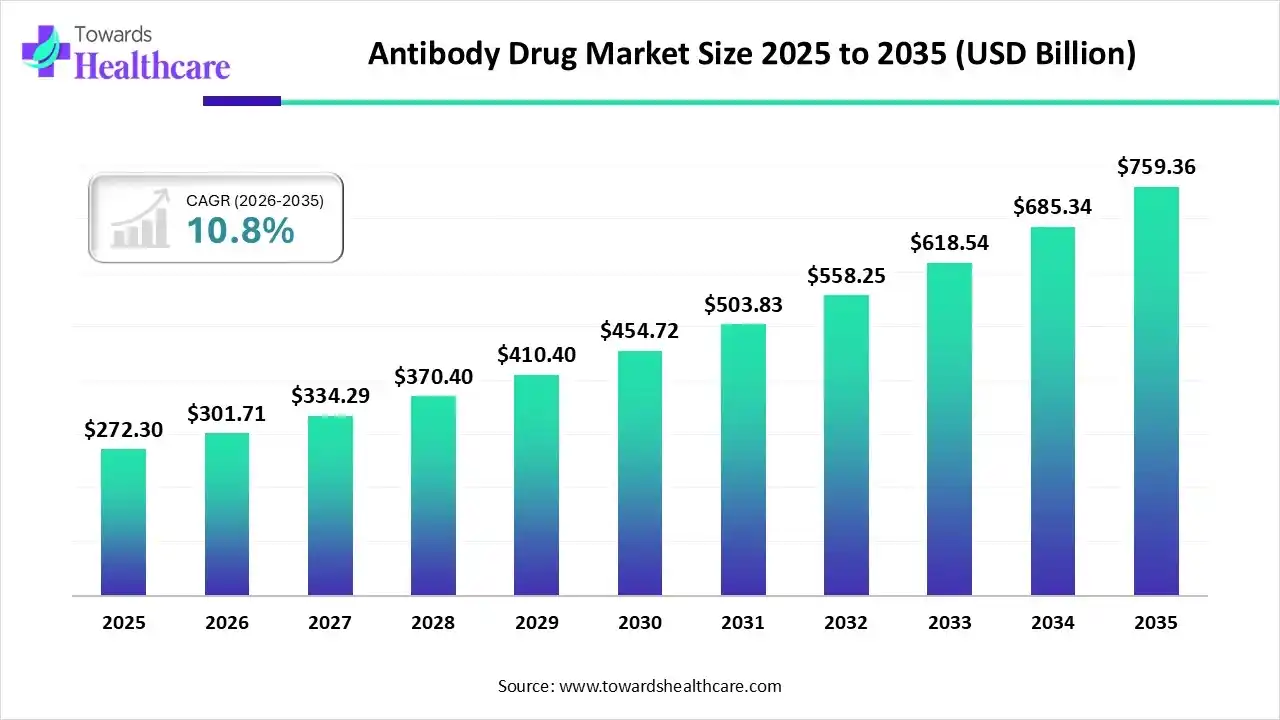
| Metric | Details |
| Market Size in 2025 | USD 272.3 Billion |
| Projected Market Size in 2035 | USD 759.36 Billion |
| CAGR (2026 - 2035) | 10.8% |
| Leading Region | North America |
| Market Segmentation | By Source, By Product, By Indication, By Distribution Channel, By Region |
| Top Key Players | AbbVie, Inc., ADC Therapeutics, AstraZeneca, Genentech, Inc., Genmab A/S, Gilead Sciences, Inc., GlaxoSmithKline, GO Therapeutics, Inc., Hiedelberg Pharma A/G, ImmunoGen, Inc., Pfizer, Inc., Takeda Pharmaceutical Company, Tallac Therapeutics, Inc. |
Monoclonal Antibodies (mAbs), which seem to be antibody preparations designed to bind to a single target, have shown potential in the fight against cancer as well as autoimmune diseases.
Monoclonal antibodies are protein-based therapies or drugs that are increasingly being used to treat chronic diseases. This blog examines the global market for antibody drug products and provides an update on their applications in various disease areas. The overall market for antibody drug products is divided into four major application areas: autoimmune diseases, solid tumors, lymphoma, and leukemia, as well as other diseases such as asthma, osteoporosis, and cardiovascular disease.
The FDA has approved more than 100 antibody products, and biologics account for roughly one-fifth of all new drug approvals each year. The presence of a healthy antibody therapy pipeline increases the company's chances of achieving higher market revenue. As a result of the high demand for antibody therapy, a surge in the development of new antibody products is expected to drive the antibody therapy market share.
Orthoclone OKT3 (muromonab-CD3) was the first licensed monoclonal antibody, approved in 1986 for use in preventing kidney transplant rejection. It is a mouse monoclonal IgG2a antibody with the cognate antigen CD3. It works by binding and inhibiting the effects of CD3 on T-lymphocytes. However, due to reported side effects, its use was restricted to acute cases (e.g. human anti-mouse antibody response).
Artificial intelligence (AI) and machine learning (ML) tools are developed to discover novel antibodies from various sources. AI and ML can accurately predict the amino acid sequences of antibodies and help researchers design antibodies based on the patient’s conditions, leading to personalized therapy development. They can also help in predicting the antigen-antibody interaction in the patient’s body, enhancing the understanding of antibody recognition. They can identify the potential mutational changes in antibodies. Additionally, AI and ML can also aid in transforming the large-scale manufacturing of antibody drugs. This leads to enhanced efficiency and reduced manual errors. Thus, AI and ML can streamline the research and manufacturing process of antibody drugs.
Immunotherapy's Expanding Applications Are Expected To Drive Up Demand For Antibody Drugs Market
Targeted therapies are intended to attack cancer cells using antibodies and chemotherapy. They are based on the idea that cancer cells have abnormal versions of certain proteins known as receptors that exist in normal cells but not in cancer cells. Antibodies against these receptors may be used in these targeted therapies. Immunotherapy is a type of cancer treatment that employs the immune system to combat the disease. It is also known as biotherapy. This type of treatment can be used alone or in conjunction with other therapies. Immunotherapy is classified as a biological therapy. Biological therapy is a type of cancer treatment that uses compounds derived from living organisms.
Rising cancer cases around the world are expected to boost demand for antibody therapy. According to the World Health Organization (WHO), cancer is the first/second leading cause of death in many countries, reducing life expectancy.
Cancer's increasing prevalence and high mortality rate have increased the demand for effective and targeted therapies such as antibody therapy. Recently, antibody-based treatments have advanced technologically by targeting tumor antigens and enhancing immune cells' anti-tumor capabilities. Furthermore, high product advancement and technologies for cancer treatment are expected to drive the antibody therapy market growth.
Market Demand For Low-Cost Bio-Similar Monoclonal Antibodies Is Growing
The popularity of the cost-effective bio-similar monoclonal antibodies market is rapidly increasing, which boosts monoclonal antibodies market growth. The goal of bio-similar antibodies is to control rising healthcare costs and to deal with economic pressure from patients and governments to reduce medication costs and improve treatment approaches. A bio-similar monoclonal antibody is 20%-25% less expensive than the original biologic drug. The number of clinical trials for a biosimilar is less than that of the original biologic drug, which explains why biosimilar drugs are less expensive.
Increasing Investment In The Development Of Novel Therapeutics
Monoclonal antibodies are gaining popularity as a safe and effective treatment for a variety of chronic diseases. Due to the success of drugs such as Remicade, Avastin, Rituxan, and Herceptin, demand for these products is rapidly increasing. Furthermore, manufacturers are engaged in the development of new drugs and product launches, which will accelerate market growth in the near future.
The U.S. FDA approved a total of 10 antibody-based drugs in 2025
|
Products
|
Companies
|
Indications
|
|
Datroway (Datopotamab deruxtecan)
|
AstraZeneca, Daiichi Sankyo
|
Breast cancer, non-small lung cancer
|
|
Anniko (Penpulimab)
|
Akeso, Sino Biopharmaceutical
|
Non-keratinizing nasopharyngeal carcinoma
|
|
Imaavy (Nipocalimab)
|
Johnson & Johnson
|
Generalized myasthenia gravis
|
|
Emrelis (Telisotuzumab vedotin-tllv)
|
AbbVie
|
Non-squamous non-small cell lung cancer
|
|
Enflonsia (Clesrovimab)
|
Merck & Co.
|
Respiratory syncytial virus
|
|
Andembry (Garadacimab-gxii)
|
CSL
|
Hereditary angioedema
|
|
Lynozyfic (Linvoseltamab)
|
Regeneron
|
Multiple myeloma
|
|
Voyxact (Sibeprenlimab-szsi)
|
Otsuka
|
Immunoglobulin A nephropathy
|
|
Exdensur (Depemokimab)
|
GSK
|
Severe asthma
|
|
Yartemlea (Narsoplimab)
|
Omeros
|
Hematopoietic stem cell transplant-associated thrombotic microangiopathy
|
The Approval Process Required Various Stringent Regulatory Policies Which Might Hinder The Market Growth
The long, as well as strict regulations for approval and launch, will be a major hurdle faced by the a7ntibody-drug market. The failure to meet the stringent regulations and results had led to the withdrawal of various clinical studies by the regulatory authorities.
The Functional Disadvantages Of Therapeutic Antibodies Are Impeding Antibody Drug Market Growth
Certain functional disadvantages of therapeutic antibodies, such as insufficient pharmacokinetics and tissue accessibility, as well as impaired interactions with the immune system, have been identified, and these deficiencies indicate areas where more research is needed. When monoclonal antibodies were first developed, they encountered serious problems when used as therapeutics. The first monoclonal antibodies were murine molecules that were recognized as foreign when injected into patients, causing the immune system to remove them. Furthermore, in order to be effective, antibodies frequently need to interact with immune system components such as receptors on effector cells or the complement cascade.
Monoclonal Antibodies Are Expensive
The mAbs are very expensive. The low financial capabilities of the low and middle-income groups in developing and underdeveloped countries, as well as a lack of proper access to healthcare facilities, pose a significant challenge to market players and may impede the growth of the global monoclonal antibodies market.
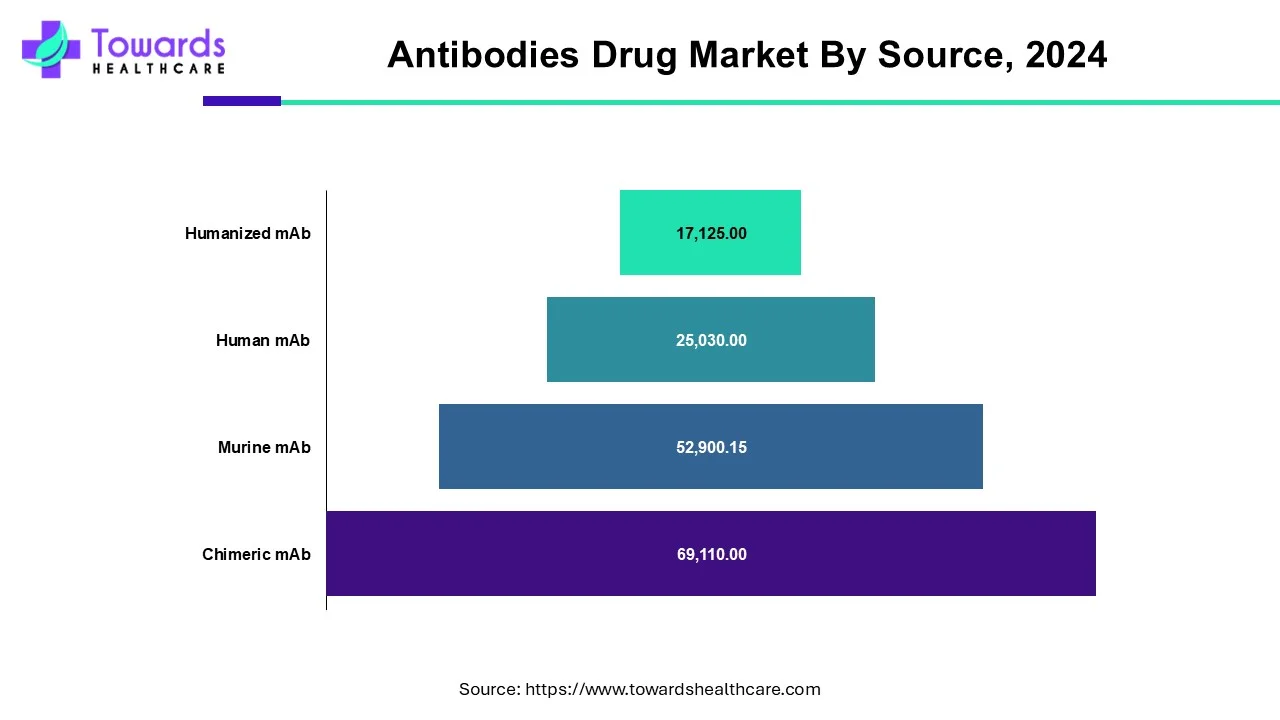
Which Source Segment Dominated the Antibodies Drug Market?
According to the source, human antibodies will dominate the global monoclonal antibody market. The introduction of Adalimumab biosimilar is anticipated to provide significant market opportunities during the forecast period. Increased pharmaceutical company investments in human mAb research, as well as increased demand for human antibodies, are propelling the market growth. Rising technological advancements to develop a molecular understanding of diseases, a rapidly aging population, and expanding applications of monoclonal antibodies are some of the factors driving the global monoclonal antibody market growth of human antibodies.
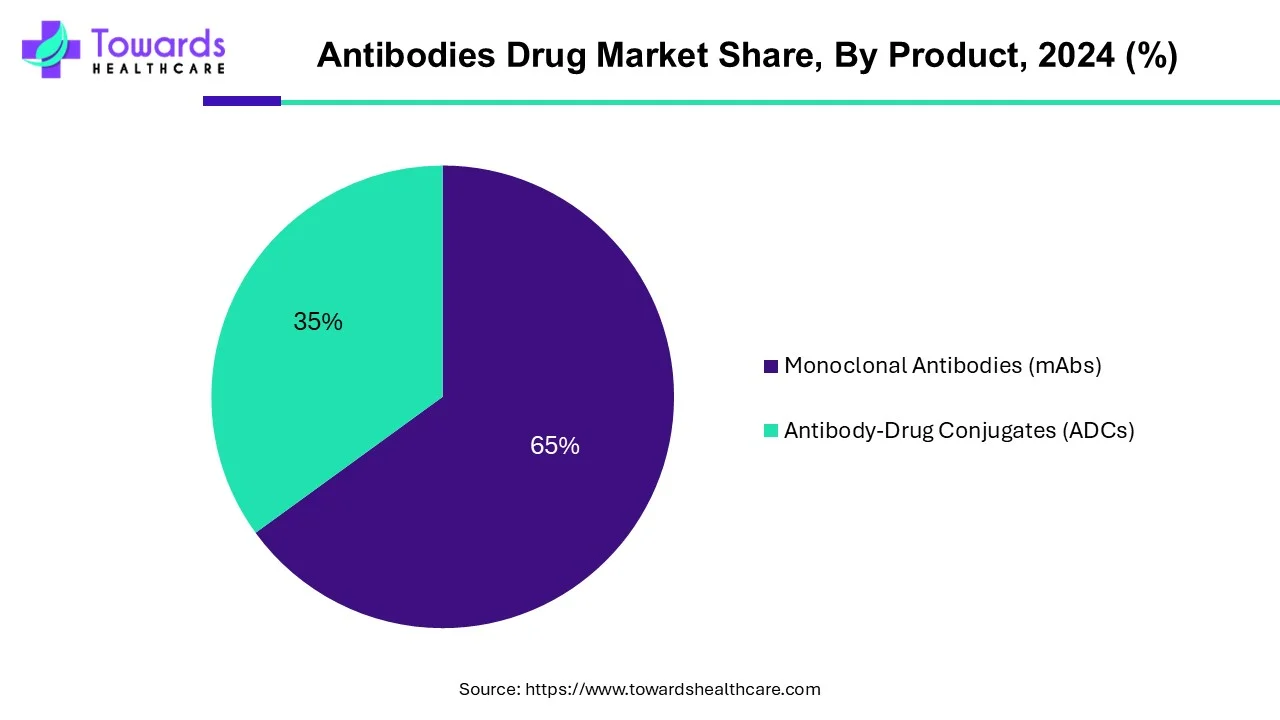
Why Did the Monoclonal Antibodies Segment Dominate the Antibodies Drug Market?
By product, the monoclonal antibodies segment led the global market in 2024. Monoclonal antibodies are laboratory-made proteins that boost the immune system to fight against various harmful pathogens such as viruses. They are produced or composed of cells derived from a single cell. They are used for various purposes, from diagnosis to treatment of numerous chronic disorders. They are used as probes to identify materials in laboratories, in home-testing kits, or to type tissue and blood for use in transplants. The rising prevalence of chronic disorders and technological advancements to extract monoclonal antibodies boost the segment’s growth.
By product, the antibody-drug conjugates segment is projected to expand rapidly in the market in the coming years. Antibody-drug conjugates (ADCs) are developed to provide targeted treatment with fewer side effects and better therapeutic action. The growing research and development activities and favorable regulatory frameworks promote the segment’s growth. Novel ADCs are designed and developed to treat numerous chronic disorders. As of June 2023, 11 ADCs have been approved by the U.S. FDA.
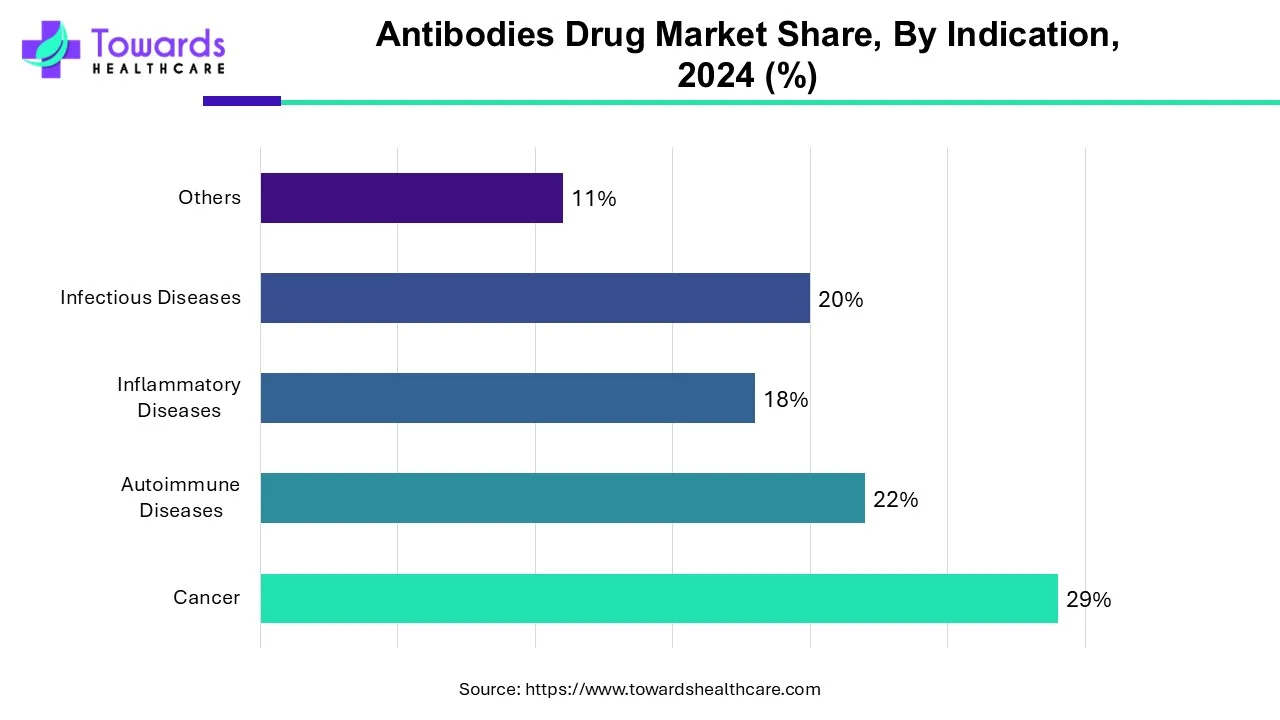
How the Cancer Segment Dominated the Antibodies Drug Market?
Cancer will dominate the global antibody drug market, according to indications. This increase is simply due to the increased prevalence of cancer and the rising demand for monoclonal antibodies in the treatment of various cancers such as lung cancer, breast cancer, colorectal cancer, and prostate cancer. The greater efficacy of mAbs in cancer treatment with few or no side effects is a major factor driving the high demand for monoclonal antibodies among cancer patients. Rising healthcare spending and increased awareness of mAbs, along with their effectiveness in cancer treatment, behold their dominance in the global market.
By indication, the autoimmune diseases segment is expected to grow at the fastest rate in the market during the forecast period. The rising prevalence of autoimmune diseases and their increasing complexity augment the segment’s growth. The growing research and development activities discover novel drug targets involved in autoimmune diseases, promoting the development of novel antibody therapeutics.
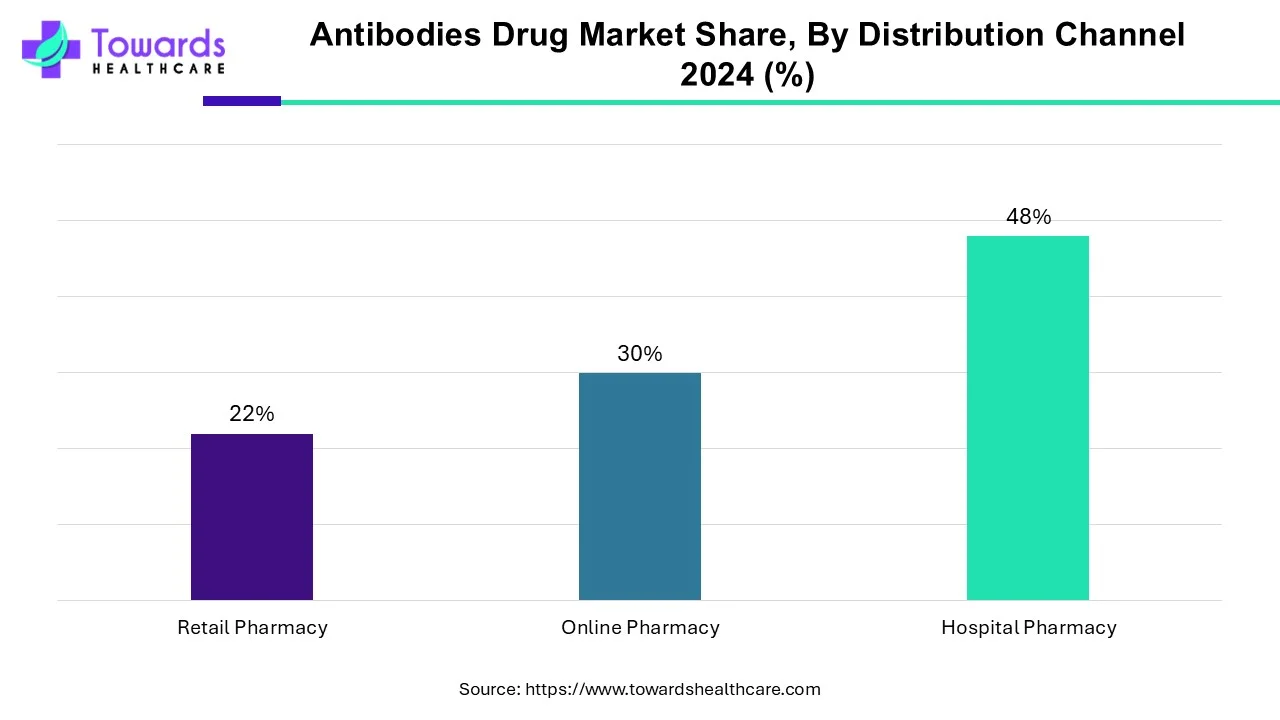
What Made Hospital Pharmacies the Dominant Segment in the Antibodies Drug Market?
In, the hospital pharmacies segment held the largest share of the antibodies drug market segment based on the distribution channel. This is due to an increase in hospital admissions due to the rising prevalence of chronic diseases such as cancer, autoimmune diseases, and rheumatoid arthritis among the population. The increased availability of advanced healthcare facilities has resulted in increased sales revenue for hospital pharmacies in the market. The wide variety of products and drugs available in hospital pharmacies makes it a convenient location for purchasing various drugs, such as mAbs.
By distribution channel, the online pharmacy segment is estimated to show the fastest growth over the forecast period. Online pharmacies offer the facility to order drugs through e-commerce websites in the comfort of their home. The segmental growth is attributed to special discounts, free home delivery, and online consultation services. Patients can order drugs from a wide range of options and compare prices of the same products from different manufacturers.
The increased prevalence of chronic diseases in North America, the presence of advanced healthcare infrastructure, a rapidly aging population, high healthcare expenditure, and increased public awareness of mAbs have resulted in increased demand for monoclonal antibodies. According to the American Cancer Society, there will be over 1.8 million new cancer cases and approximately 606,520 deaths in the United States in 2020. By 2060, the US population is expected to have approximately 24% geriatric people aged 65 or older.
Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period. The rising prevalence of chronic disorders and increasing investments drive the market. Favorable government support to expand the biopharmaceutical sector boosts the market. The suitable regulatory frameworks potentiate market growth driven by NMPA approval of novel antibody therapeutics in China. The Indian government announced an investment of Rs. 15,000 crore under the PLI scheme from FY2022-23 to FY2027-28 for the manufacturing of pharmaceutical products such as Patented/Off-Patented drugs, biopharmaceuticals, complex generics, etc. The presence of suitable manufacturing infrastructure also augments the market. The Japanese government laid out a five-year plan to promote drug development. It also encouraged pharmaceutical companies to start clinical trials on much-needed drugs by 2026 and create at least 10 drug discovery startups by 2028.
Europe is a notable region in the antibody drug market due to various factors such as high prevalence of cancer, strong research and development and manufacturing capabilities, and beneficial regulatory landscapes. The increasing prevalence of solid tumors across European countries is also contributing to the growth of the antibody drug market. The region is experiencing significant growth in antibody drug development and applications due to numerous research institutions and pharmaceutical companies like BioNTech actively involved in antibody drug development, especially in cancer therapies and immunological treatments.
UK Market Trends
It is estimated that approximately 3.5 million people are living with cancer, and an additional 3.5 million people are living with a rare disease in the UK. Hence, the UK government and regulatory agencies are at the forefront of supporting antibody drug development. In June 2025, the MHRA approved serplulimab (Hetronifly) to treat adults with extensive-stage small cell lung cancer (ES-SCLC).
Latin America is expected to grow at a notable CAGR in the foreseeable future. The growing demand for biologics, the rising prevalence of chronic disorders, and increasing research activities boost the market. The burgeoning biopharma sector and the presence of key players foster market growth. Researchers develop biosimilars to enhance the accessibility and affordability of antibody drugs for the local population. Regulatory agencies update their standards for the evaluation and approval of antibody biosimilars.
Brazil Market Trends
Companies like Recepta Biopharma, Biocon Biologics, and Eurofarma are major manufacturers and distributors of antibody drugs in Brazil. Inventions in the biotech sector in Brazil are patented to protect the innovation. In 2024, the Brazilian Patent and Trademark Office (BPTO) recorded a total of 27,701 patent applications. This is due to a surge in R&D investments by 2% annually.
The Middle East & Africa are expected to grow at a considerable CAGR in the upcoming period. Government organizations launch initiatives to establish a suitable research and manufacturing infrastructure for the development of antibody drugs. The evolving regulatory landscape and increasing demand for personalized medicines augment the market. People are becoming aware of screening and early diagnosis of genetic and rare disorders, allowing healthcare professionals to provide early intervention.
UAE Market Trends
The UAE government recently established a new pharmaceutical law, the Emirates Drug Establishment (EDE), for the regulation of pharmaceuticals, medical devices, and biological products. The increasing prevalence of rare diseases also potentiates the need for antibody drugs. It is estimated that about 1 in 17 people, or 7% of the total population suffer from a rare disease in the UAE.
Explore how top players are reshaping the Antibodies Drug Market: https://www.towardshealthcare.com/companies/antibodies-drug-companies
Companies are currently funding clinical trials for over 570 mAbs. Approximately 90% of these are early-stage studies designed to assess patient populations' safety (Phase I) or safety and preliminary efficacy (Phase I/II or Phase II). The majority of mAbs in Phase I (70%) are for cancer treatment, and the proportions of mAbs in Phase II and late-stage clinical studies (pivotal Phase II, Phase II/III, or Phase III) are similar.
The latest research focuses on developing better linkers and payloads and generating novel drugs for expanded applications.
Key Players: Takeda Pharmaceuticals, AbbVie, and AstraZeneca
Trials are conducted to investigate novel linkers and conjugation technologies to improve stability, reduce off-target toxicity, and enhance tumor penetration.
Key Players: Merck, QPS, Regeneron, and Roche
Patient support refers to providing enhanced access to novel antibody drugs and financial assistance.
Wyatt McDonnell, CEO and CO-founder of Infinimmune, commented in an interview that the company aims to become the single source of a safer and better generation of antibody therapeutics. He said that the company is currently building a pipeline of drugs and partnering with pharmaceutical companies to advance antibody programs together.
By Source
By Product
By Indication
By Distribution Channel
By Region
February 2026
February 2026
February 2026
February 2026