February 2026

The global advanced therapies in biopharmaceutical medicine market is emerging as a high-growth sector, with strong potential to generate significant revenue in the coming years from 2025 to 2034. This upward momentum is driven by rapid technological innovation, increasing demand for personalized treatments, and shifting healthcare priorities that are transforming the landscape of modern medicine.
The advanced therapies in biopharmaceutical medicine are providing curative or long-term therapeutic benefits, which in turn, are increasing their use. Moreover, advancements in these therapies for discovering new possibilities for disease treatment, along with the use of AI, are increasing. The use of AI helps in enhancing the therapeutic potential, quality, as well as development process. Additionally, the advancing healthcare, innovation, collaborations, as well as product launches are also increasing their use. Thus, all these factors are responsible for enhancing the advanced therapies in biopharmaceutical medicine market.

Innovative class of medicinal products that are characterised by advanced technologies is known as advanced therapy medicinal products (ATMPs). These advanced therapies in biopharmaceutical medicine are used to provide a cure or long-term therapeutic benefit to the patient by targeting the tissue, cellular, or genetic-based abnormalities in a disease. Furthermore, in certain cases, the use of these therapies can help in correcting the genetic defect as well. Moreover, advancements in therapies such as tissue engineering, cell therapy, gene therapy, and their hybrid forms are opening new possibilities for the treatment of various diseases.
The AI provides various advantages to overcome limitations such as patient-specific variability, limited space, decentralized manufacturing, and limited staffing in the advanced therapies in the biopharmaceutical medicine market. At the same time, it helps in automated analysis, which improves productivity. Moreover, the use of AI in the development of personalized advanced therapies is also being considered to enhance the therapeutic potential as well as the quality standards. Additionally, to improve the safety and efficacy of these therapies use of AI is also increasing.
Growing Diseases
The increasing incidence of diseases is increasing the demand for advanced biopharmaceutical therapies. In some cases, diseases such as cancer, rare diseases, heart disease, etc, may not be effectively treated with the use of traditional medications; thus, the use of advanced therapies in biopharmaceutical medicine increases. These therapies offer targeted action that minimizes the side effects, enhancing the treatment approach. Similarly, they can be used to replace or repair defective genes or proteins, increasing their use in genetic diseases. This drives the advanced therapies in biopharmaceutical medicine market growth.
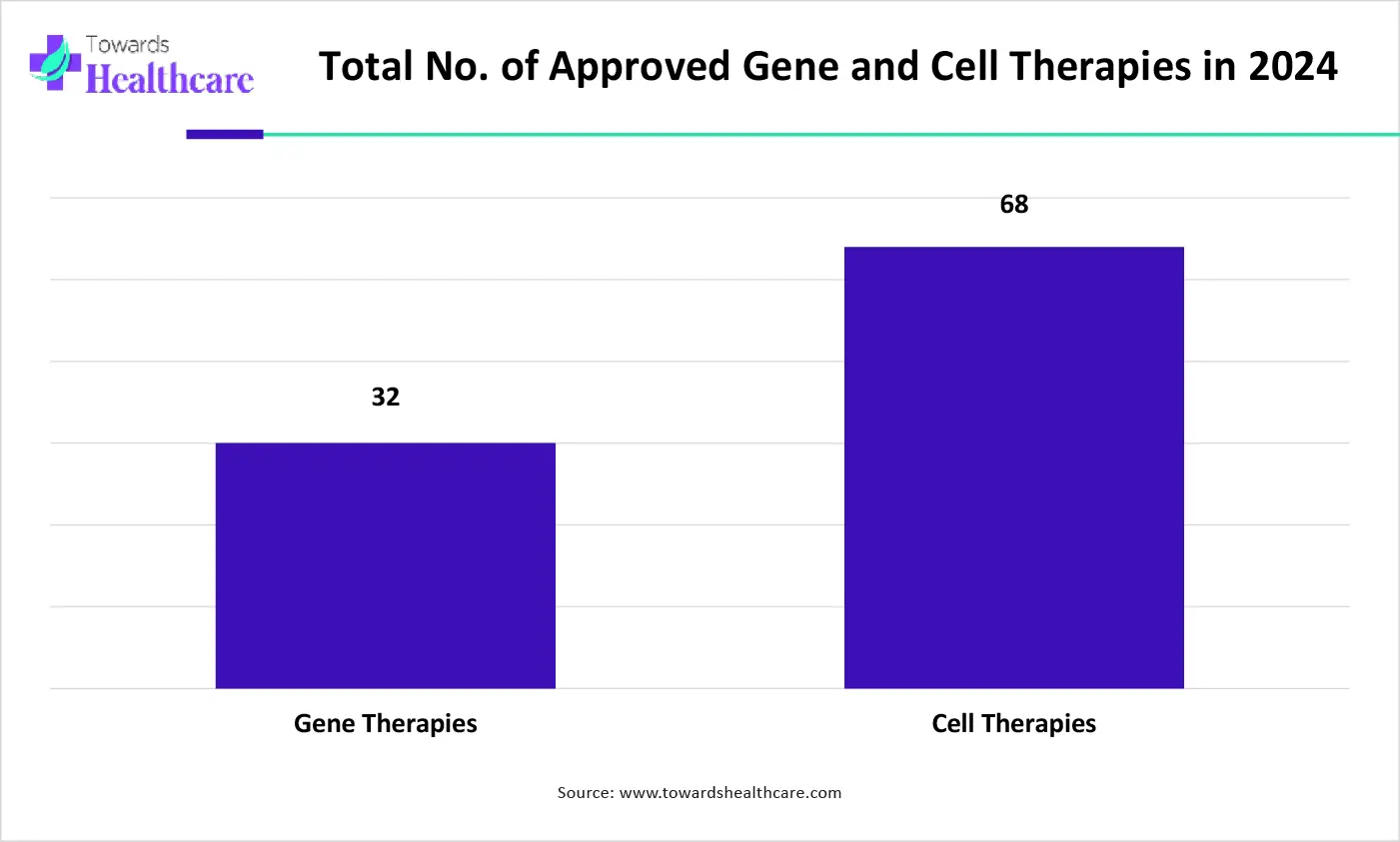
The graph represents the total number of approved gene and non-genetically modified cell therapies in 2024. It indicates that there is a rise in the development of new advanced therapies in biopharmaceutical medicine for the effective management of growing diseases. Thus, this in turn will ultimately promote the market growth.
High Prices
The cost associated with advanced therapies in biopharmaceutical medicine is high. This, in turn, affects patients in the low to middle-income countries. At the same time, the accessibility is also hampered, which in turn limits the acceptance rates of these therapies. Thus, high prices of advanced therapies in biopharmaceutical medicine may restrict the market growth.
Why is Increasing Gene Editing Therapies an Opportunity in the Advanced Therapies in Biopharmaceutical Medicine Market?
The use of gene editing therapies is increasing due to the growing genetic diseases. Hence, with the use of these gene editing therapies, newer and effective solutions are being provided to such diseases. At the same time, personalized treatment options are also being developed with the help of gene editing therapies. Furthermore, research and development in diseases other than genetic diseases are also being conducted to enhance the treatment approaches. Thus, all these factors are promoting the advanced therapies in biopharmaceutical medicine market growth.
For instance,
By therapy type, the gene therapy segment held the largest share in the market in 2024. The use of gene therapy in various genetic disorders increased as it provided a long-term effect or even cured the diseases. Thus, this contributed to the market growth.
By therapy type, the CAR-T segment is expected to show the highest growth at a notable CAGR during the upcoming years. The growing cancer cases is increasing the use of CAR-T treatment approaches.
By application type, the oncology segment led the market in 2024. The use of advanced therapies in the field of oncology increased due to its growing incidence and rising demand. Furthermore, the use of personalized medication also contributed to the same.
By application type, the cardiovascular diseases segment is expected to show the fastest growth rate during the predicted time. Growing innovations in ATMPs, to treat cardiovascular diseases, are increasing their demand in the market.
By product type, the tissue-engineered products segment held the dominating share in the market in 2024. The tissue-engineered products were used in various diseases, as they showed a wide range of applications, which enhanced the market growth.
By product type, the combined ATMPs segment is expected to show the highest growth during the forthcoming period. The use of combined ATMPs is rapidly increasing, as their use in complex diseases is growing due to enhanced effectiveness.
By distribution channel type, the hospital pharmacies segment led the market in 2024. The hospital pharmacies distributed a variety of advanced therapies, which were safely formulated and stored in specialized storage conditions.
By distribution channel type, the online pharmacies segment is expected to show the fastest growth rate during the upcoming years. The use of online pharmacies is growing as they are enhancing the convenience of the patient, as well as providing at-home deliveries.
By end user, the hospital segment held the largest share in the global market in 2024. The hospitals provided various advanced therapies with the help of specialized staff, which enhanced patient safety and outcomes. This promoted the market growth.
By end user, the clinics segment is expected to show the highest growth during the predicted time. The clinics are offering cost-effective treatment approaches with novel advanced therapies, which are attracting patients.
North America dominated the advanced therapies in biopharmaceutical medicine market in 2024. The presence of skilled personnel as well as advanced technologies made the healthcare sector in North America well-developed. This increased development of advanced therapies in biopharmaceutical medicine contributed to the market growth.
The growing use of advanced technologies in the U.S. is increasing new development in advanced therapies in biopharmaceutical medicine. At the same time, rising incentives and policies are enhancing their use.
The growing interest in advanced therapies in biopharmaceutical medicine is enhancing the industries in Canada to increase innovations in these therapies, which in turn, is also encouraging the research conducted.
Asia Pacific is expected to host the fastest-growing advanced therapies in biopharmaceutical medicine market during the forecast period. The increasing development in biopharmaceutical medicines and therapies, as well as rising investments in Asia Pacific, is enhancing the market growth.
The industries in China are adopting various advanced technologies, which in turn, are improving the development as well as the production process of these advanced therapies. At the same time, new polices are also being implemented to make these therapies more accessible.
The increasing diseases in India are increasing the use and demand for advanced therapies in biopharmaceutical medicines. Furthermore, to make these therapies affordable, investments are being provided by the government as well as the private sector.
Europe is expected to grow significantly in the advanced therapies in biopharmaceutical medicine market during the forecast period. The growing research and development in Europe in advanced therapies in biopharmaceutical medicine, which is supported by the government and regulatory bodies, is promoting the market growth.
Germany consists of well-developed healthcare sectors where the growing research and development in industries, as well as institutes, is increasing the innovations in advanced therapies. Moreover, growing biotech industries and startups are further driving these developments.
With the help of a robust healthcare system in the UK, the use and development of advanced therapies in biopharmaceutical medicines are being effectively enhanced. Furthermore, the support of regulatory bodies and the government is encouraging new development and clinical trials.
By Therapy Type
By Application
By Product Type
By Distribution Channel
By End-User
By Region
February 2026
January 2026
January 2026
January 2026