Revenue, 2025
1.6 Billion
Forecast, 2035
8.86 Billion
AI in Regulatory Affairs Market Size and Company Landscape with Regional Insights
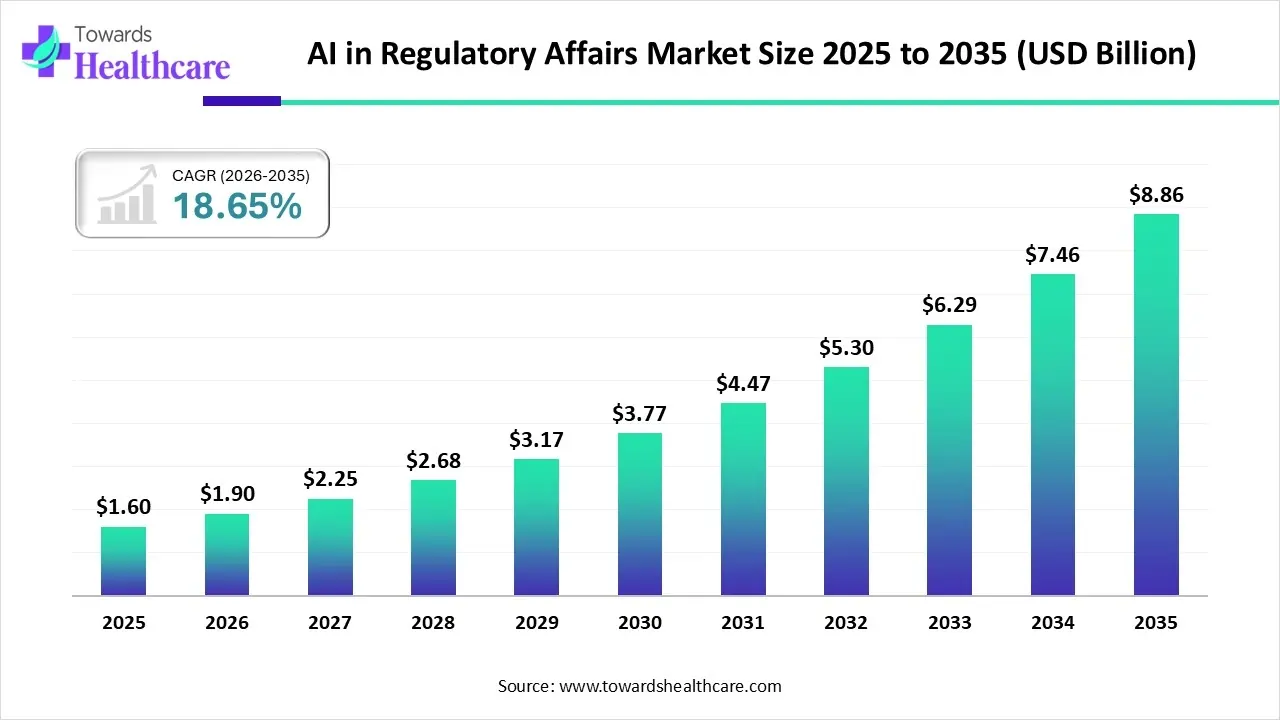
The global AI in regulatory affairs market size is calculated at US$ 1.6 billion in 2025, grew to US$ 1.9 billion in 2026, and is projected to reach around US$ 8.86 billion by 2035. The market is expanding at a CAGR of 18.65% between 2026 and 2035.
Key Takeaways
- The AI in regulatory affairs market will likely exceed USD 1.6 billion by 2025.
- Valuation is projected to hit USD 8.86 billion by 2035.
- Estimated to grow at a CAGR of 18.65% starting from 2026 to 2035.
- The North America AI in regulatory affairs market held the largest revenue share in 2024.
- Asia Pacific is expected to grow at the fastest CAGR during the forecast period.
- By component, the software/platforms segment led the AI in regulatory affairs market with the largest market share in 2024.
- By component, the services segment is expected to grow at the fastest CAGR during the forecast period.
- By deployment mode, the cloud-based segment accounted for the largest revenue share in 2024 and is expected to grow at the fastest CAGR during the forecast period.
- By application, the regulatory intelligence segment accounted for the largest revenue share in 2024.
- By application, the pharmacovigilance & safety reporting segment is expected to grow at the fastest CAGR during the forecast period.
- By end-use, the pharmaceutical companies segment held the largest market share in 2024.
- By end-use, the CRO/CDMO segment is expected to grow at the fastest CAGR during the forecast period.
| Key Elements |
Scope |
| Market Size in 2025 |
USD 1.6 Billion |
| Projected Market Size in 2035 |
USD 8.86 Billion |
| CAGR (2025 - 2035) |
18.65% |
| Leading Region |
North America |
| Market Segmentation |
By Component, By Deployment Mode, By Application,By End-use, By Region |
| Top Key Players |
IQVIA, Freyr Solutions, DDReg Pharma, RegASK, Deloitte (via its RegAI solution), IBM, Oracle, Microsoft, Google (Alphabet), Tempus AI, Accenture, Wipro, Zenovel, Innoplexus, Workiva, ComplyAdvantage, MetricStream, Viz.ai, ArisGlobal, Veeva Systems |
The AI in regulatory affairs market is expanding due in large part to the increasing complexity of regulatory submissions and the growing cost pressures in drug development and regulatory operations. Further driving market expansion are technological developments and growing cost pressures in drug development and regulatory operations. The AI in regulatory affairs industry is driven by the growing volume and complexity of international regulatory requirements in the pharmaceutical, biotechnology, and medical device industries.
What is the Role of AI in Regulatory Affairs?
There is more to integrating AI and machine learning into regulatory affairs than just small efficiency gains. It denotes a paradigm shift in how regulatory bodies function, communicate with health authorities, and deliver strategic value to pharmaceutical development. Every facet of the regulatory function is being transformed by these technologies, from AI-assisted safety signal detection to predictive regulatory intelligence and automated document generation.
AI in Regulatory Affairs Market Outlook
- Industry Growth Overview: The market is anticipated to grow significantly due to the need for improved compliance monitoring, automation of repetitive tasks, and increased efficiency. The growing complexity and volume of international regulations in the life sciences industry are the direct cause of this expansion.
- Global Expansion: Because AI can automate manual tasks, increase efficiency, and improve compliance with a variety of intricate international regulations, the market for AI in regulatory affairs is growing globally. As businesses look for quicker drug approvals and real-time regulatory intelligence, its use in North America, Europe, and the Asia-Pacific is growing.
- Major Investors: Leading venture capital firms like Andreessen Horowitz (a16z), Sequoia Capital, Lightspeed Venture Partners, and Khosla Ventures are major investors in the AI in regulatory affairs market. Additionally, corporate investors like Microsoft and Google Ventures (GV) are heavily involved in the market.
Segmental Insights
Component Insights
Why the Software/Platforms Segment Dominated the Market in 2024?
The software/platforms segment led the AI in regulatory affairs market with the largest AI in regulatory affairs market share in 2024. The proper tools and software are essential resources that promote efficiency and guarantee compliance in the complex field of regulatory affairs. These specialized tools, ranging from regulatory intelligence platforms to document management systems, are essential for tracking regulatory changes, maintaining accurate records, and managing the complexity of regulatory submissions.
Services
The services segment is expected to grow at the fastest CAGR in the AI in regulatory affairs market during the forecast period. To ensure that pharmaceutical, biotechnology, and medical device companies comply with all laws and regulations governing the creation, approval, production, and marketing of their products, regulatory affairs services encompass a broad range of professional support activities. These services are essential to the successful registration and lifecycle management of healthcare products in international markets.
Deployment Mode Insights
Why Cloud-Based Segment Dominated the Market in 2024?
By deployment mode, the cloud-based segment accounted for the largest revenue share of the AI in regulatory affairs market in 2024 and is expected to grow at the fastest CAGR during the forecast period. By improving scalability, flexibility, and cost-efficiency, cloud computing integration in pharmaceutical and life sciences regulatory submissions is revolutionizing drug development. Cloud platforms make it easier to manage data, store clinical trial datasets securely and scalably, and share, integrate, and analyze data to support well-informed decision-making. By facilitating smooth communication and data exchange, these platforms enhance international cooperation between researchers, regulators, and healthcare providers.
On-Premises
The on-premises segment is expected to grow significantly during the forecast period. Policies and regulations, data security, and technological advancements are driving the ongoing growth of the on-premises regulatory management systems market. The need for compliance management systems has been fueled by governments around the world imposing stricter regulations on the production and distribution of drugs.
Application Insights
Which Application Dominated the Market in 2024?
The regulatory intelligence segment accounted for the largest revenue share of the AI in regulatory affairs market in 2024 because of the constantly changing regulatory environment, it can be difficult to make important decisions, such as portfolio expansion and international market entry, in the complex world of biopharmaceuticals and life sciences. In this situation, regulatory intelligence, or RI, is essential. It employs technology to drive growth, increase revenue, and add value while simplifying complexities.
Pharmacovigilance & Safety Reporting
The pharmacovigilance & safety reporting segment is expected to grow at the fastest in the AI in regulatory affairs market CAGR during the forecast period. By supervising pharmaceutical products throughout their whole lifecycle, from development to post-market monitoring, regulatory affairs play a critical role in guaranteeing drug safety and efficacy. Regulatory affairs specialists collaborate closely with health authorities, including the FDA and EMA, to guarantee that pharmaceuticals pass stringent safety requirements and undergo extensive clinical testing before being released onto the market.
Data Migration & Integration
The data migration & integration segment is expected to grow significantly during the forecast period. In the life sciences, data migration and integration play a complex and vital role. The ability to smoothly transfer data across complex ecosystems is a critical success factor as organizations work to innovate, collaborate, and deliver better patient care.
End-Use Insights
Why did the Pharmaceutical Companies Dominate the Market in 2024?
The pharmaceutical companies segment held the largest AI in regulatory affairs market share in 2024. Of all the industries, the pharmaceutical sector is the most regulated. They are in charge of keeping up with the growing breadth and complexity of regulations and serve as the company's main point of contact with organizations like the FDA. Regulatory affairs specialists in the pharmaceutical sector are knowledgeable about clinical research procedures as well as the legal and regulatory frameworks.
CRO/CDMO
The CRO/CDMO segment is expected to grow at the fastest CAGR during the forecast period. By using their knowledge to guarantee compliance with international health authorities (such as the FDA and EMA) throughout the drug development lifecycle, CROs and CDMOs offer crucial regulatory affairs support.
Biotechnology Companies
The biotechnology companies segment is expected to grow significantly in the AI in regulatory affairs market during the forecast period. Recombinant proteins, cell and gene therapies, and monoclonal antibodies are examples of biotechnology products that are frequently intricate and inventive. In order to move these products from research and development to market approval, regulatory affairs specialists in the biotech industry are essential in navigating the constantly changing regulatory environment.
Regional Growth

- North America dominated the AI in regulatory affairs market in 2024.
- Asia Pacific is estimated to host the fastest-growing AI in regulatory affairs market.
- Europe is expected to grow at a significant CAGR in the AI in regulatory affairs market.
- South America is expected to grow significantly in the AI in regulatory affairs market.
- The Middle East and Africa are expected to grow at a lucrative CAGR in the AI in regulatory affairs market.
Presence of Entities like the FDA and Health Canada Drives North America
North America dominated the AI in regulatory affairs market in 2024. Market expansion is fueled by the complexity and volume of regulatory requirements from organizations like Health Canada and the U.S. FDA. Furthermore, flexible, scalable regulatory solutions are required due to the expansion of clinical trials and the advancement of personalized medicine. AI and data analytics integration enhances compliance management and real-time monitoring. Furthermore, the need for economical regulatory function outsourcing speeds up market expansion.
Regulating America: OIRA's Oversight and FDA's Health
OIRA is the central body of the U.S. government that oversees the coordination of federal privacy policy, the approval of government information gathering, the establishment of government statistical procedures, and the review of Executive Branch regulations. Furthermore, the U.S. Food and Drug Administration (FDA) is a health authority that oversees the use of experimental medications in the U.S.
For instance,
- In June 2025, nearly a month ahead of schedule, the US Food and Drug Administration (FDA) unveiled a new artificial intelligence (AI) tool that the agency claims greatly enhances operations and aims to modernize its functions. The agency said it will continue to add capabilities based on staff feedback.
Asia Pacific Regulatory Affairs: Advancements Spark Market Opportunity
Asia Pacific is estimated to host the fastest-growing AI in regulatory affairs market during the forecast period, driven by recent regulatory developments and the rapidly growing number of clinical studies and trials. The market's expansion has been fueled by the region's massive cost-cutting initiative. This region's enormous supply of high-quality labor has greatly expanded the market. Another factor contributing to the market expansion is the rapidly growing geriatric population.
Drug Approval in China: Regulatory Affairs and Strategic Insights
China has the second-largest pharmaceutical market in the world, thanks to increased access to new medications, changing laws, and rising public demand for pharmaceuticals. Drugs, medical devices, and traditional medicines are regulated by key agencies such as the Ministry of Human Resources and Social Security (MOHRSS), the National Medical Products Administration (NMPA), and the National Health Commission (NHC).
Europe is expected to grow at a significant CAGR in the AI in regulatory affairs market during the forecast period, driven by the region's growing need for CROs, the presence of major pharmaceutical companies, and ongoing drug discoveries. Several CROs focused on drug discovery have set up centers in countries such as the UK, Germany, and France, taking advantage of these nations' strong research infrastructure and highly qualified labor force.
MHRA Marketing Authorisation: UK Regulatory Approvals
According to the GOV.IN the UK, the Medicines and Healthcare Products Regulatory Agency (MHRA) oversees regulatory matters. This entails overseeing applications for clinical trials, variations, and marketing authorizations, as well as making sure goods like pharmaceuticals, medical equipment, and blood components adhere to safety, quality, and efficacy requirements.
South America is expected to grow significantly in the AI in regulatory affairs market during the forecast period. As South American businesses use digital platforms to automate compliance, the market for AI in regulatory affairs is growing. Effective regulatory support is necessary for expanding clinical trials and introducing new products. AI-driven tools are used by outsourcing partners to improve documentation accuracy, expedite submissions, and monitor risks in real time.
Brazil's AI Regulatory Strategy Advances
Brazil's market is driven by expanding AI adoption in sectors like healthcare and finance. The nation's new Brazilian Artificial Intelligence Plan supports AI research and development. Proposed legislation on AI governance, including risk assessment, is creating a formalized regulatory framework for sophisticated AI tools.
MEA Region Driving Regulatory Digitalization
The Middle East and Africa are expected to grow at a lucrative CAGR in the AI in regulatory affairs market during the forecast period. The Middle East and Africa (MEA) region is experiencing strong growth, fueled by the rising complexity of advanced biologics and medical devices. Companies are increasingly outsourcing regulatory functions, leading to the rapid adoption of AI-enabled technologies. This shift supports streamlined submissions and proactive compliance monitoring.
GCC Pushes Smart Regulation for Growth
Gulf Cooperation Council (GCC) countries, notably Saudi Arabia and the UAE, see high demand for AI-driven compliance solutions. Investments in smart city initiatives and digital transformation are critical drivers. AI tools help automate processes like dossier compilation and ensure timely, efficient regulatory approvals for expanding product portfolios.
Top Companies in the AI in Regulatory Affairs Market

- IQVIA
- Freyr Solutions
- DDReg Pharma
- RegASK
- Deloitte (via its RegAI solution)
- IBM
- Oracle
- Microsoft
- Google (Alphabet)
- Tempus AI
- Accenture
- Wipro
- Zenovel
- Innoplexus
- Workiva
- ComplyAdvantage
- MetricStream
- Viz.ai
- ArisGlobal
- Veeva Systems
Top Vendors in the AI in Regulatory Affairs Market & Their Offerings
| Company |
Offerings |
Contributions |
Highlights |
Impact |
| Freyr Solutions |
AI submissions automation |
Speeds approvals using smart workflows globally |
Expanding global support rapidly |
Enhances compliance efficiency overall |
| DDReg Pharma |
Automated dossier review tools |
Improves accuracy across regulatory tasks significantly |
Strong regional presence growing |
Reduces review timelines effectively |
| RegASK |
AI monitoring dashboards |
Delivers timely global intelligence consistently |
Multilingual insights driving clarity |
Strengthens regulatory foresight proactively |
| Deloitte RegAI |
Intelligent compliance engines |
Modernizes enterprise regulatory processes comprehensively |
Integrated ecosystem enabling trust |
Supports scalable governance sustainably |
| IBM |
NLP-driven regulatory analytics |
Supports data-led decisions across industries strategically |
Broad automation capabilities are advancing |
Improves operational resilience holistically today |
Company 1: Veeva Systems
Company Overview:
- Veeva is the global leader in cloud software for the life sciences industry, offering a comprehensive suite of applications across R&D, Quality, Regulatory, and Commercial operations.
- Its flagship product for this area, Veeva Vault RIM, is a widely adopted Regulatory Information Management system that heavily integrates AI for content classification, data extraction, and process automation.
Corporate Information:
- Headquarters: Pleasanton, CA, USA
- Year Founded: 2007
- Ownership Type: Public (NYSE: VEEV)
History and Background:
- Founded by former Salesforce executives, Veeva initially focused on CRM for pharmaceutical sales.
- It rapidly expanded into the R&D and Quality space, launching the Veeva Vault platform to manage content and data with a strong focus on GxP and regulatory compliance.
- The transition to a Public Benefit Corporation in 2021 underscored its commitment to its life sciences mission and community.
Key Milestones/Timeline:
- 2007: Company founded, launches Veeva CRM on the Salesforce platform.
- 2013: IPO on the NYSE.
- 2015: Launches Veeva Vault Regulatory Suite (RIM, Submissions, Publishing).
- 2023-2024: Significant integration of Generative AI (GenAI) capabilities across the Vault platform, including Vault RIM.
Business Overview:
- Veeva operates the Industry Cloud for life sciences, providing software, data, and business consulting to streamline the drug development and commercialization lifecycle.
- Fiscal Year 2024 Total Revenues were $2,363.7 million.
Business Segments/Divisions:
- Veeva Commercial Solutions: Includes CRM, commercial content management, and data products.
- Veeva R&D and Quality Solutions (Vault Platform): Includes Clinical, Regulatory (RIM), Quality, Safety, and Medical solutions. This segment is the primary driver for AI in regulatory affairs.
Geographic Presence:
- Global, serving over 1,000 customers worldwide, including the largest pharmaceutical companies and emerging biotechs. Strong presence in North America and Europe.
Key Offerings:
- Veeva Vault RIM Suite: Cloud-based Regulatory Information Management, Submissions, and Publishing solutions.
- Veeva Vault AI: AI-powered functionality embedded across the Vault platform for content automation, data extraction, and compliance checking.
- Veeva Link: Data products providing intelligence on key opinion leaders and regulatory bodies.
End-Use Industries Served:
- Pharmaceutical and Biopharmaceutical Companies
- Medical Device and Diagnostics Companies
- Biotechnology Companies
- Contract Research Organizations (CROs) and Contract Manufacturing Organizations (CMOs)
Key Developments and Strategic Initiatives:
- Mergers & Acquisitions: Generally focuses on organic growth, with occasional acquisitions of smaller, specialized technology firms to enhance platform capabilities.
- Partnerships & Collaborations:
- Veeva AI Partner Program (Apr 2024): Provides partners with advanced technology and support to integrate GenAI solutions with Veeva Vault applications.
- Ongoing collaborations with leading global consulting firms to deploy Veeva Vault and AI features.
- Product Launches/Innovations: Continuous embedding of AI/GenAI into the Vault platform to automate routine regulatory tasks like document metadata extraction, content generation assistance, and quality checks.
- Capacity Expansions/Investments: Significant ongoing investment in its cloud platform infrastructure and R&D for AI innovation.
- Regulatory Approvals: Provides validated, GxP-compliant systems for customers to meet FDA, EMA, and other global regulatory requirements.
Distribution Channel Strategy:
- Primarily direct sales model to large enterprise customers.
- Strong ecosystem of consulting partners (system integrators) who implement and configure the Veeva Vault platform.
- Cloud-based, subscription services model.
Technological Capabilities/R&D Focus:
- Core Technologies/Patents: Veeva Vault Platform (a multi-tenant, cloud-based architecture), proprietary data models for life sciences content, and domain-trained AI models for regulatory affairs.
- Research & Development Infrastructure: Dedicated R&D teams focused on platform evolution and AI integration.
- Innovation Focus Areas: Generative AI for regulatory content and submissions, unifying clinical and regulatory data, and enhancing the Vault platform's low-code configurability.
Competitive Positioning:
- Strengths & Differentiators: Deep life sciences domain expertise, single unified cloud platform (Vault) for all R&D and commercial needs, and a large, established customer base (high switching costs).
- Market presence & ecosystem role: Dominant market leader in the Regulatory Information Management (RIM) software space, setting the industry standard for cloud-based regulatory systems.
SWOT Analysis:
- Strengths: Deep industry focus, highly scalable cloud platform, strong customer loyalty.
- Weaknesses: High reliance on the life sciences sector, potential competition from large tech firms entering the space.
- Opportunities: Rapid adoption of GenAI in regulatory processes, expansion into adjacent regulated industries (e.g., CPG, Chemicals).
- Threats: Increased regulatory scrutiny of AI/ML models, evolving global data privacy laws.
Recent News and Updates:
- Press Releases: Veeva Announces Fourth Quarter and Fiscal Year 2025 Guidance (Feb 2025 - projected FY25 revenues of $2,725 - $2,740 million). Focus on AI integration across the Vault suite.
- Industry Recognitions/Awards: Consistently recognized as a leader in life sciences R&D and Quality management software by major analyst firms.
Company 2: IQVIA Inc.
Company Overview:
- IQVIA is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry.
- Their AI regulatory solutions are delivered through their Technology & Analytics Solutions segment and within their Contract Research Organization (CRO) service offerings, leveraging massive, proprietary datasets.
Corporate Information:
- Headquarters: Research Triangle Park, NC, USA
- Year Founded: 2017 (Formed by the merger of Quintiles and IMS Health)
- Ownership Type: Public (NYSE: IQV)
History and Background:
- Formed from the merger of Quintiles (a global CRO) and IMS Health (a global provider of healthcare data and analytics).
- This combination created a "Human Data Science" powerhouse, linking clinical trial execution with real-world data and commercial insights.
- The company has made significant investments in AI/ML capabilities, primarily through its IQVIA Connected Intelligence platform.
Key Milestones/Timeline:
- 2017: Merger of Quintiles and IMS Health to form IQVIA.
- 2019: Launches the Orchestrated Clinical Trials platform, heavily featuring AI/ML for trial design and execution, with implications for regulatory submissions.
- 2024: Continues to heavily integrate Generative AI for faster data synthesis, clinical data quality, and regulatory documentation across its services.
Business Overview:
- IQVIA provides end-to-end solutions that blend proprietary data, technology, and domain expertise.
- Its regulatory AI capabilities are embedded in both its software solutions and its outsourced regulatory affairs services.
- FY 2024 Total Revenues: $15.8 billion (estimated/projected).
Business Segments/Divisions:
- Technology & Analytics Solutions (TAS): Offers cloud-based software, data, and analytics, including its Regulatory and Safety intelligence platforms.
- Research & Development Solutions (R&DS): Provides outsourced clinical research services, including clinical trial management, lab services, and embedded regulatory support.
- Contract Commercial & Principal Primary Solutions (CCPPS): Commercialization services.
Geographic Presence:
- Global footprint, operating in over 100 countries. Offers regulatory intelligence covering all major global health authorities (FDA, EMA, PMDA, etc.).
Key Offerings:
- IQVIA RIM Smart Suite: Regulatory Information Management system that leverages AI for regulatory intelligence.
- IQVIA Connected Intelligence: Integrated platform blending R&D, commercial, and real-world data with AI/ML to drive decision-making.
- AI-Driven Regulatory Outsourcing Services: Comprehensive regulatory strategy, submission, and publishing services using proprietary AI tools.
End-Use Industries Served:
- Pharmaceutical and Biopharmaceutical Companies (largest customer base)
- Medical Device and Diagnostics Companies
- Government Agencies and Public Health Organizations
Key Developments and Strategic Initiatives:
- Mergers & Acquisitions: Historically active in M&A to consolidate data assets and clinical capabilities. Focus on acquiring specialized data and technology firms.
- Partnerships & Collaborations:
- Continuous strategic partnerships with global healthcare systems and data providers to enrich its real-world data (RWD) assets, which in turn fuel its regulatory intelligence AI models.
- Product Launches/Innovations: Ongoing development of predictive analytics and GenAI tools within its TAS segment to forecast regulatory submission timelines and identify regulatory risks.
- Capacity Expansions/Investments: Substantial investment in its AI and RWD infrastructure to maintain a competitive advantage in data-driven services.
Distribution Channel Strategy:
- Direct sales through its global clinical and commercial teams.
- Service-led distribution where AI-enabled tools are bundled and delivered as part of its R&D and Regulatory Outsourcing services.
- Cloud-based, subscription model for its software products (TAS).
Technological Capabilities/R&D Focus:
- Core Technologies/Patents: The IQVIA Connected Intelligence Platform, proprietary Real-World Data (RWD) assets (petabytes of non-identified patient data), and advanced AI/ML algorithms for predictive modeling.
- Research & Development Infrastructure: Extensive R&D team and data science labs focused on human data science and AI applications.
- Innovation Focus Areas: Using Generative AI for accelerated regulatory writing, predictive modeling for safety and compliance risk, and leveraging RWD for regulatory submissions.
Competitive Positioning:
- Strengths & Differentiators: Unmatched scale and integration of clinical services, technology, and proprietary data (RWD), which is a key input for regulatory AI.
- Market presence & ecosystem role: The largest global CRO and a leading technology provider, giving it a central, influential role across the drug development and regulatory ecosystem.
SWOT Analysis:
- Strengths: Massive proprietary data assets, global service delivery capability, deep expertise in both clinical and regulatory operations.
- Weaknesses: Potential complexity from managing two large predecessor companies (Quintiles/IMS), high competition in the CRO space.
- Opportunities: Leveraging its RWD for novel AI-driven regulatory pathways (e.g., synthetic control arms), growing demand for outsourced, data-driven regulatory services.
- Threats: Data privacy and security regulations in new markets, competition from technology-focused pure-play software vendors.
Recent News and Updates:
- Press Releases: Regularly announces new RWD partnerships and AI innovations focused on accelerating clinical development, which directly impacts regulatory submissions.
- Industry Recognitions/Awards: Often cited as a leader in CRO services, RWD, and Life Science IT by industry analysts.
Recent Developments in the AI in Regulatory Affairs Market
- In October 2025, the first vertical agentic AI command center for regulatory affairs will be introduced by RegASK, the leading agentic AI platform that is revolutionizing workflow orchestration and regulatory intelligence.
- In September 2025, PharmaPendium AI, a generative AI assistant for regulatory intelligence in drug development, was introduced by Elsevier, a multinational information and analytics company.
- In August 2025, the beta release of its AI-powered Regulatory Assistant within Cortellis Regulatory Intelligence was announced by Clarivate Plc, a prominent worldwide supplier of transformative intelligence.
Segments Covered in the Report
By Component
- Software/Platforms
- Services
By Deployment Mode
By Application
- Regulatory Intelligence
- Data Migration & Integration
- Dossier Management
- Document Management
- Product Registration & Approvals
- Pharmacovigilance & Safety Reporting
- Regulatory Submissions & Publishing
- Others
By End-use
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Companies
- CRO/CDMO
- Others
By Region
-
- North America
- U.S.
- Canada
- Mexico
- Rest of North America
- South America
- Brazil
- Argentina
- Rest of South America
- Europe
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
- Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
- MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA




