February 2026

The in vivo CAR-T platform market is rapidly advancing on a global scale, with expectations of accumulating hundreds of millions in revenue between 2025 and 2034. Market forecasts suggest robust development fueled by increased investments, innovation, and rising demand across various industries.
The in vivo CAR-T platform market is primarily driven by the growing demand for CAR-T cell therapy. This aligns with the increasing need for personalized medicines, especially for genetic and rare disorders. Government organizations also support the development of CAR-T cell therapy through funding and initiatives. The integration of artificial intelligence (AI) revolutionizes the functionality of the platform. The future looks promising, with the development of novel delivery systems.
The in vivo CAR-T platform market refers to the emerging field of in vivo chimeric antigen receptor T-cell (CAR-T) therapy, where T cells are genetically modified directly inside the patient’s body, rather than being extracted, engineered ex vivo, and reinfused as in traditional CAR-T therapies. These platforms use nanoparticles, viral vectors, lipid-based delivery systems, or gene-editing payloads (e.g., mRNA, DNA, CRISPR) to deliver CAR constructs to T cells in situ. This next-generation approach promises faster treatment timelines, lower costs, off-the-shelf scalability, and reduced manufacturing complexities, making CAR-T more accessible for both hematologic and solid tumors.
AI can improve the market by facilitating the development of an in vivo CAR-T platform by introducing automation. Bioinformatics is an emerging tool that leverages AI and machine learning (ML) algorithms to predict the effect of CAR-T cells in humans. It enables the development of a suitable delivery system for targeted delivery with reduced systemic side effects. AI and ML can also predict the outcomes of the in vivo CAR-T cell therapy platform within the body. They can also aid in modifying the release of CAR-T cells.
CAR-T Cell Therapy Development
The major growth factor for the in vivo CAR-T platform market is the increasing development of CAR-T cell therapy. The growing geriatric population and rapidly changing demographics potentiate the demand for personalized medicines. CAR-T cell therapy is a type of immunotherapy mainly used for different types of blood cancer. Numerous government and private organizations provide funding for developing novel CAR-T cell therapies and conduct clinical trials. The increasing number of clinical trials is a result of growing research activities. There were a total of 1,587 clinical trials registered on the clinicaltrials.gov website related to CAR-T cell therapy as an intervention.
High Production Cost
The development of an in vivo CAR-T platform requires a high investment in its manufacturing. This is due to their patient-specific nature and complex manufacturing process. The average manufacturing cost of a CAR-T cell therapy is around $300,000 to $500,000.
What is the Future of the In Vivo CAR-T Platform Market?
The market future is promising, driven by the development of novel delivery platforms. Advancements in nanotechnology enable the development of nanoparticles to deliver CAR-T cells within the human body. Researchers focus on creating affordable and efficient nanoparticle-based gene editing may be a promising strategy to produce CAR-T cells in vivo. Nanoparticles can improve their stability and improve their transfection rate due to their small size and customized constituents. Moreover, the liposomal drug delivery system is one of the emerging technologies to address solubility and bioavailability.
By delivery vector/technology, the lipid nanoparticles (LNPs) segment held a dominant presence in the market in 2024. This is due to the growing demand for nanomedicines and the ability to protect against RNA degradation. Lipid nanoparticles (LNPs) ensure homogenous and consistent nanoparticles with demonstrated stability and longer shelf-life. They eliminate the difficulty with bioavailability and solubility, allowing them to penetrate the complex structures of human cells easily.
By delivery vector/technology, the polymer nanoparticles segment is expected to grow at the fastest CAGR in the market during the forecast period. Different polymers are used to deliver CAR-T cell therapy in humans. The availability of natural and synthetic polymers offers a wide range of options for the delivery system. Biodegradable polymers are preferred to cause negligible harm to patients. Polymer nanoparticles have the ability to protect the genetic cargo during delivery.
By target indication, the hematologic malignancies segment held the largest revenue share of the market in 2024. This is due to the rising prevalence of blood cancers and the availability of FDA-approved therapeutics. By 2030, the global prevalence of hematologic malignancies is projected to reach approximately 4.63 million. As of 2025, a total of 7 CAR-T cell therapies are approved. All these seven therapies are approved for hematologic malignancies.
By target indication, the solid tumors segment is expected to grow with the highest CAGR in the market during the studied years. Researchers are focusing on the development of in vivo CAR-T cell therapies for the treatment of solid tumors. Recent research studies have found that in vivo, genetically modified CAR-T cells can reduce tumor size by 30% and increase survival rates by 50% compared to conventional CAR-T cells.
By payload type, the mRNA-encoded CARs segment contributed the biggest revenue share of the market in 2024 and is expected to expand rapidly in the market in the coming years. The demand for mRNA-encoded CAR-T cells is increasing due to their potential safety compared to those transduced with viral CARs. mRNA-encoded CARs mitigate the side effects of viral CARs. Advancements in genomic and RNA technologies facilitate the development of mRNA-encoded CARs. Moreover, mRNA technology offers high simplicity, low cost, and an acceptable safety profile. This technology also enables large-scale manufacturing of CAR-T cells.
By end-user/application area, the biopharma R&D segment led the market in 2024. The segmental growth is attributed to favorable research infrastructure and suitable capital investment. Biopharma companies have skilled professionals to research novel CAR-T cell therapies. The increasing number of biopharma startups raises market competition, resulting in new product launches. This enables companies to strengthen their market position and stay ahead in the competitive field.
By end-user/application area, the personalized medicine providers segment is expected to witness the fastest growth in the market over the forecast period. The growing demand for personalized medicines and favorable regulatory policies encourage personalized medicine providers to provide innovative therapeutics. They have suitable infrastructure and specialized equipment to conduct research and manufacture these medicines based on patients’ conditions.
By stage of development, the preclinical platforms segment accounted for the highest revenue share of the market in 2024. The growing number of preclinical trials to study the efficacy and toxicity in animal models propels the segment’s growth. Numerous studies have shown promising anti-tumor activities of the in vivo CAR-T cell platform in preclinical trials. Several delivery systems or vectors are assessed by introducing CAR-T cells in animal models. Stringent regulatory requirements also necessitate preclinical study reports.
By stage of development, the Phase I clinical trials segment is expected to show the fastest growth over the forecast period. Phase I clinical trials are conducted in a small human population of about 20-100 healthy volunteers. They are sometimes conducted with severely or terminally ill patients with cancer. They are conducted to assess dosing regimen, side effects, and pharmacokinetic properties.
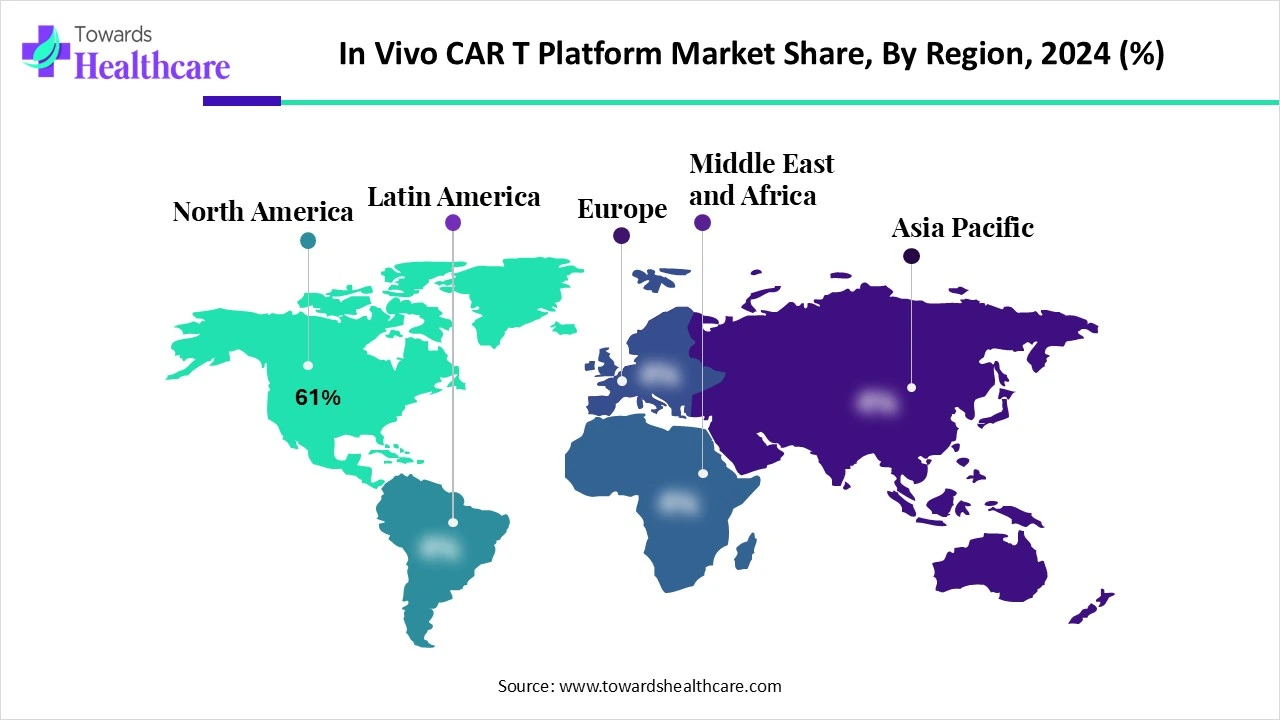
North America dominated the market share by 61% in 2024. The availability of state-of-the-art research and development facilities, the presence of key players, and increasing investments are the major growth factors for the market in North America. Government organizations launch initiatives to support the development of personalized medicines and provide funding. The increasing number of clinical trials also favors market growth.
Key players, such as Capstan Therapeutics, Moderna, Inc., and Geneos Therapeutics, are the major contributors to the market in the U.S. The U.S. FDA approved a total of 16 novel personalized medicines in 2023. The U.S. conducts the highest number of clinical trials globally. As of July 2025, 508 studies have been registered on the clinicaltrials.gov website related to CAR-T cell therapy.
As of July 2024, five CAR-T cell therapies have been approved by the U.S. FDA and Health Canada. Additionally, 6 of 10 Canadian provinces have authorized CAR-T products for public reimbursement. Researchers from the National Research Council Canada (NRC) focus on creating novel made-in-Canada CAR-T cell therapies with expertise in producing antibodies.
Asia-Pacific is expected to grow at the fastest CAGR in the in vivo CAR-T platform market during the forecast period. The market is mainly driven by supportive government policies and localized biotech innovation. Countries like China, Japan, and South Korea are at the forefront of developing cell and gene therapy due to sufficient funding from the government and private organizations. Research institutions conduct seminars, workshops, and conferences to share the recent updates in the field and train individuals on the latest technologies.
In China, the prevalence of hematologic malignancies is estimated to reach 859,652 by 2030. As of July 2025, the National Medical Products Administration (NMPA) approved 6 CAR-T cell therapies for 9 indications. The Chinese government optimized its foreign investment environment to allow foreign players to set up their cell and gene therapy manufacturing facilities.
There are currently 5 CAR-T cell therapies in Japan, including Kymriah, Yescarta, Breyanzi, Abecma, and Carvykti, for the treatment of hematologic malignancies. Japan had registered 25 clinical trials as of July 2025 for CAR-T cell therapies.The demand for CAR-T cell therapies is increasing in Japan with the expansion of medical facilities and a gradual increase in eligible patients.
Europe is expected to grow at a notable CAGR in the in vivo CAR-T platform market in the foreseeable future. The increasing investments and collaborations among key players and research institutions foster market growth. The burgeoning biopharma sector and the rising number of startups also contribute to market growth. The rising prevalence of cancer and rare disorders necessitates researchers to develop novel CAR-T cell platforms.
In May 2024, the German Cancer Aid awarded the Leibniz Institute for Immunotherapy (LIT) and the University Hospital Regensburg (UKR) a 2.6 million euro grant to support an innovative clinical study using stem-like CAR-T cells to treat patients with advanced lymphomas. Researchers aim to improve the survival chances of patients who relapse with lymphoma.

Stephen J. Russell, CEO of Vyriad, Inc., commented that the in vivo approach to CAR-T cell therapies has the potential to transform the field. The company believes that its targeted delivery platform, lentiviral vector platform, can enable this vision for the industry, and its collaboration with Novartis represents an exciting step forward in advancing the next generation of CAR-T cell treatments.
By Delivery Vector/Technology
By Target Indication
By Payload Type
By End-User/Application Area
By Stage of Development
By Region
February 2026
February 2026
February 2026
February 2026
