February 2026

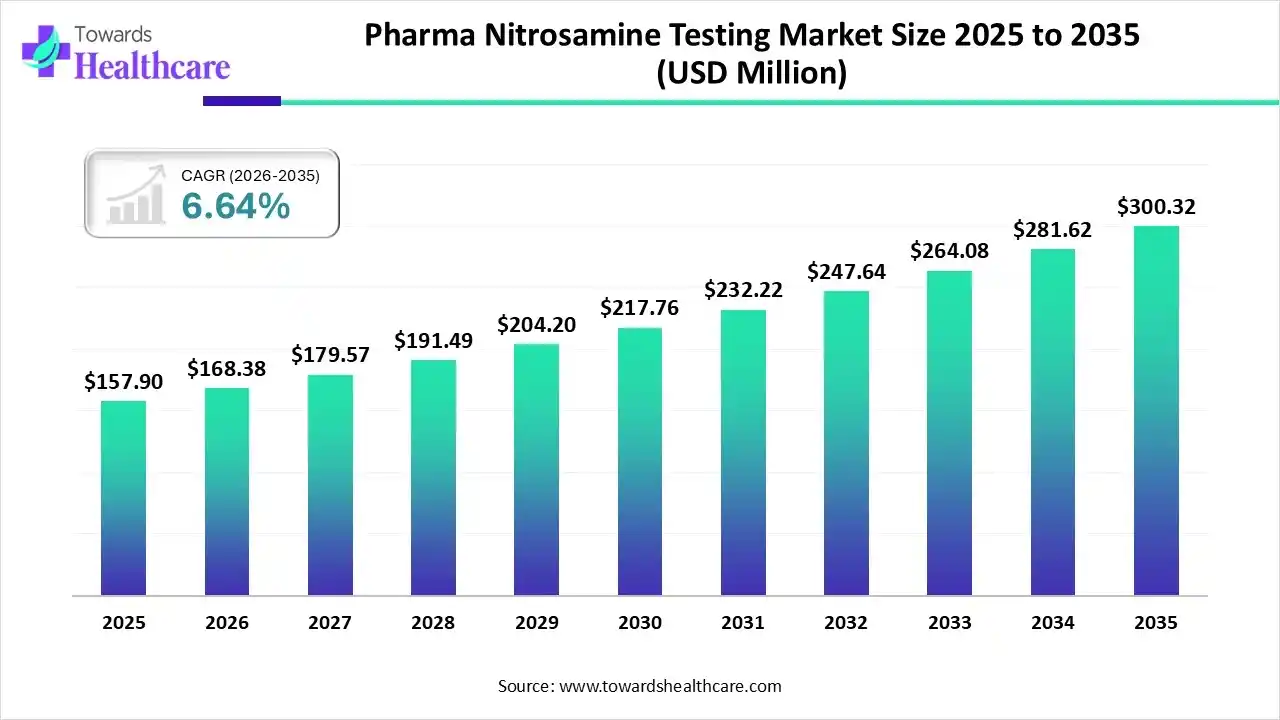
The pharma nitrosamine testing market size was valued at US$ 157.9 million in 2025 and is projected to grow to 168.38 million in 2026. Forecasts suggest it will reach approximately US$ 300.32 million by 2035, registering a CAGR of 6.64% during the period.
The pharma nitrosamine testing market is expanding rapidly due to increasing government scrutiny and growing challenges to the safety of medicinal products. Government agencies like EMA and FDA mandate guidelines for identifying nitrosamine impurities, which is driving the growth of the market.
In North America, growing technological advancement in impurity identification methods and growing trends in outsourcing testing services increases the demand for the market. While in the Asia Pacific region, the increasing focus of pharmaceutical companies on risk mitigation and quality assurance of medicinal products drives the growth of the market.
| Key Elements | Scope |
| Market Size in 2026 | USD 168.38 Million |
| Projected Market Size in 2035 | USD 300.32 Million |
| CAGR (2026 - 2035) | 6.64% |
| Leading Region | North America |
| Market Segmentation | By Testing Type, By Product Type, By Sample Type, By End User, By Region |
| Top Key Players | SGS, Thermo Fisher Scientific, EKG Life Science Solutions, LLC (EKG Labs), Eurofins BioPharma Product, Ampac Fine Chemicals DBA Ampac Analytical, Waters Corporation, Nucro-Technics, Boston Analytical, Selvita, KYMOS Group, Alcami Corporation |
The pharma nitrosamine testing market is growing rapidly due to the testing of nitrosamine impurities, is plays a significant role in the pharmaceutical sector because of the toxic nature of these compounds. Consistent scrutiny ensures that drug products are below the risk-defined exposure limits, and it protects the patients from possible carcinogenic effects. Nitrosamine compounds play a significant role in the pharmaceutical field, serving as both a tool for ensuring product safety and a means of understanding and controlling the manufacturing process. This testing is performed to determine if their medicinal product contains nitrosamine impurities. It detects and quantifies nitrosamine traces in raw materials, medicinal products, packaging, and active pharmaceutical ingredients (APIs).
AI integration in pharma nitrosamine testing is driving the growth of the market as AI-based technology in nitrosamine risk assessment provides an innovative approach to evaluating, identifying, and modifying potential nitrosamine impurities in healthcare products. This advanced methodology has been gaining momentum as government authorities, with the FDA and EMA, highlight the necessity of advanced risk assessment frameworks to confirm patient safety. AI-driven technology can process massive datasets, detect patterns, and make predictions, making it an influential supporter in addressing nitrosamine challenges. AI-based technology evaluates manufacturing workflows to detect conditions conducive to nitrosamine formation. It advises substitute reagents, solvents, or reaction conditions to reduce risks.
Increasing Healthcare Advancement
Growing scientific advancements and complex healthcare products have reshaped the healthcare sector, offering advanced solutions and tools for managing a wide range of conditions, from acute viral infections to chronic diseases. As science progresses, developing gradually complex pharmaceutical products, manufacturers and biotech companies must evolve their tools, strategies, and procedures to keep up with the rate of scientific discovery, so growing worldwide production of APIs and finish drugs specifically generic drugs, growing the challenges of impurities, which drives the demand for pharma nitrosamine, contributes the growth of nitrosamine testing market.
Challenges in Pharma Nitrosamine Testing
Many challenges with nitrosamine identification are the increasingly low sensitivities being required by regulatory agencies. This needs the use of techniques like liquid chromatography–tandem mass spectrometry (LC–MS/MS) technology. These instruments support achieving the target sensitivities, but reproducibility rates and robustness may be a challenge to the market growth.
Increasing Advancement in Analytical Techniques
Current development in analytical techniques has transformed the healthcare industry, allowing more efficient, accurate, and comprehensive characterization of drug substances and products. Many modern analytical processes are being applied for the analysis of nitrosamines, like high-performance liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry, which have huge sensitivity and selectivity, creating the opportunity for the pharma nitrosamine testing market.
By testing type, the LC-MS/MS (Liquid Chromatography–Mass Spectrometry) segment held the largest share of the market in 2024 and is expected to fastest growing over the forecast period in the pharma nitrosamine testing market in 2024, as LC-MS assays speed up various analytes in a single analytical run along with minimal incremental expenses. This has a significant impact to simplify the laboratory setup and offers additional useful data. LC-MS approaches are also being developed for quantitative, sensitive, multiplexed assays of plasma proteins and peptides. LC-MS offers extreme sensitivity and specificity as compared to the direct injection technique. With the development of cost-effective and reliable instruments, LC-MS is starting to play a significant role in various areas of clinical biochemistry and compete with conventional liquid chromatography and other techniques like immunoassay.
By product type, the services segment is dominant and expected to grow at the fastest CAGR in the pharma nitrosamine testing market, as the nitrosamine analysis plays a significant role when it comes to screening medicinal products and gaining the interest of consumers. Nitrosamine testing services for finished drug products confirm that the finished product delivered to patients is free from detectable levels of the impurities. Nitroamine impurities testing services allow for the quantification and identification of nitroamine traces in medicinal products, APIs, and raw materials. Nitrosamine analysis services for the medicinal sector to overcome challenges such as low detection limits of nitrosamine impurities and complex samples.
By sample type, the drug substances (APIs) segment is dominant in the pharma nitrosamine testing market, as nitrosamine impurity testing supports drug substances manufacturers in producing contaminant-free products and confirming their high quality. Quick access to high-quality toxicity information lessens the requirement to carry out needless testing in the API development process.
The drug products (finished dosage forms) segment is expected to be the fastest-growing in the market due to the applications of modern analytical procedures like GC-MS, HPLC-MS, and LC-TOF, precisely identify the presence of nitrosamines in drug products. Nitrosamine testing for finished drug products confirms that the end product delivered to patients is free from harmful levels of these impurities. Assimilating nitrosamine analysis for finished drug products in consistency protocols aids in detecting delayed impurity formation.
By end user, the pharmaceutical & biotech companies segment is dominant in the pharma nitrosamine testing market, as it provides a fast, solvent-free, and consumer-friendly method, and it offers an effective substitute to conventional chromatographic techniques. This solution is the best choice for routine impurity testing, confirming that manufacturers stay ahead of government requirements while improving efficiency. It supports drug manufacturers in navigating a shifting regulatory landscape. Nitrosamine analysis has a significant role to play when it comes to the transmission of pharmaceutical products.
The contract research organizations (CROs) segment is expected to be the fastest-growing in the market due to CROs improving clinical trials with low expenses, compliance, expertise, and faster timelines, enhancing global research. CROs play a significant role in ensuring the quality and safety of pharmaceutical products by testing for nitrosamines and ensuring that they are within acceptable limits.
North America dominated the pharma nitrosamine testing market, as the growing early detection initiative of pharmaceutical manufacturing through screening improves survival and diminishes mortality by finding chronic diseases at an early stage. These initiatives help faster identification of safety concerns, ensure compliance with evolving standards, and contribute to the market. Growing medical research and development (R&D) in North America. Also, the strong presence of healthcare research labs and modern analytical testing capabilities is driving the growth of the market.
In the United States, increasing pharmaceutical manufacturing is growing the demand for advanced testing solutions, including nitrosamine testing. The industry also generates economic activity in healthcare sectors when it purchases goods and services as inputs for the production and development of its products. The advancement of an analytical procedure is a measure of its capacity to remain unaffected by minor, but deliberate variations in process parameters, and offers an indication of its reliability during normal usage, which drives the growth of the market.
In 2025, the Government of Canada will invest more than $2.3B in 41 projects in the vaccine, biomanufacturing, and therapeutics ecosystem, consolidate domestic pandemic response capabilities, and life science innovation. This investment helps enhance productivity and create future economic growth. Healthcare manufacturing remains a significant part of the economic infrastructure of Canada. Pharmaceutical production facilities help regional economies in Canada and contribute directly to national manufacturing growth, which drives the growth of the pharma nitrosamine testing market.
The Asia Pacific region is projected to experience the fastest growth in the pharma nitrosamine testing market during the forecast period due as the pharmaceutical sector is essential in delivering and developing medications which save lives and enhance health results. Despite facing several risks, it continues to transform with advancements in science and technology, which increases the demand for pharma nitrosamine testing. The presence of advanced research, expansion of manufacturing capabilities, and the speed-up of the commercialization of novel drugs are driving the growth of the market.
China's healthcare industry is undergoing a profound revolution, shaped by growing demographics, evolving healthcare requirements, and government reforms, driving the growth of the market. Increasing urbanization, an aging population, and an increasing middle class are driving increased demand for healthcare solutions, creating opportunities for the growth of the pharma nitrosamine testing market. With a rapid advancement in technology and a growing ageing demographic, China is experiencing a surging demand for medical care services, pharmaceuticals, and medical devices that must meet international safety standards, including pharma nitrosamine testing demand.
India’s testing and diagnostic laboratory sector plays a momentous role in medical care by providing significant services for disease monitoring, detection, and management. This sector is a vital component of the healthcare system, providing critical services that allow the management and identification of diseases, which helps in the rapid adoption of testing services. Increasing government investment in supporting labs and pharmaceutical quality infrastructure, which contributes to the growth market.

In May 2025, Greg Behar, Recipharm’s CEO, stated, “Recipharm and PLG announced partnership; this partnership brings together Recipharm’s development and manufacturing expertise, with PLG’s 2,000 government experts, operating in 150 countries. By integrating scientific, regulatory, operational, and commercial strategy from the outset, we help our customers move faster, avoid costly delays, and build confidence that their product submissions will meet and exceed agency needs, for critical medicines worldwide.” (Source - BioSpace)
By Testing Type
By Product Type
By Sample Type
By End User
By Region
February 2026
February 2026
February 2026
January 2026