January 2026

The advanced liver cancer treatment drugs market is rapidly advancing on a scale, with expectations of accumulating hundreds of millions in revenue between 2025 and 2034. Market forecasts suggest robust development fueled by increased investments, innovation, and rising demand across various industries.
The growing incidence of liver cancer is increasing the demand for advanced liver cancer treatment drugs globally. This, in turn, is increasing their R&D, clinical trials where AI is also being used for their optimization. Moreover, the growing healthcare investments, expanding healthcare, increasing awareness, and government initiatives are increasing their use across different regions, where new products are also being launched by the companies, promoting the market growth.
The advanced liver cancer treatment drugs market is driven by growing innovation in targeted therapy and immunotherapy. The advanced liver cancer treatment drugs encompass the development, manufacturing, and commercialization of pharmacologic therapies used to treat advanced stages of liver cancer, primarily hepatocellular carcinoma (HCC), as well as cholangiocarcinoma and other rare liver malignancies. The market includes targeted therapies (tyrosine kinase inhibitors, multi-kinase inhibitors), immune checkpoint inhibitors (PD-1/PD-L1, CTLA-4), monoclonal antibodies, combination regimens (immunotherapy + targeted therapy), gene therapy candidates, and adjunct supportive therapies.
The integration of AI in the oncology sector is driving the advanced liver cancer treatment drugs market as it helps in improving the efficacy and accuracy of the treatment and diagnostic approaches. With its use, the liver cancer drugs can be optimized, and personalized medications can also be developed with improved effectiveness. Real-time and accurate information provided by AI also helps in the development and screening of the most suitable drug candidate, which in turn increases their use, improving the patient outcomes.
In October 2025, C2S-Scale 27B, which is a new artificial intelligence model, was developed by a collaboration between Google and Yale University. This model helps in predicting new drug candidates for cancer treatment by understanding the "language" of individual cells.
By drug class/mechanism of action type, the tyrosine kinase inhibitors (TKIs) segment held the dominating share of approximately 35% in the advanced liver cancer treatment drugs market in 2024, due to first-line therapy adoption. Moreover, their widespread guideline recommendations increased their use in targeting multiple liver cancer pathways. They were also used in combination with other drugs, which also contributed to their increased use.
By drug class/mechanism of action type, the immune checkpoint inhibitors segment is expected to show the fastest growth rate during the upcoming years. Due to their superior survival benefits, their use is increasing. Moreover, their enhanced efficacy, growing approvals, and improved safety profile are increasing their acceptance rates.
By treatment modality type, the oral targeted therapies segment led the advanced liver cancer treatment drugs market with approximately 40% share in 2024, as they enhanced the convenience, promoting patient comfort. At the same time, they were preferred as first-line treatment options due to their established efficacy. Moreover, their wide range of availability and multi-target action also increased their use.
By treatment modality type, the intravenous immunotherapy segment is expected to show the highest growth during the upcoming years. Due to their long-lasting action and activation of the patient's immune system against cancer, their use is increasing as a first treatment option. Additionally, their growing combinations and innovations are also enhancing the market growth.
By end user/customer type, the hospitals/oncology clinics segment held the largest share of approximately 55% in the global advanced liver cancer treatment drugs market in 2024, driven by the majority of systemic therapy administration. They also offered advanced therapy and combination therapies to the patients. Additionally, the emergency treatments and reimbursement policies were also provided to the patients.
By end user/customer type, the contract research organizations (CROs)/clinical trial sites segment is expected to show the fastest growth rate during the predicted time. They are providing support to the immunotherapy and novel combination trials. Furthermore, the growing innovations and outsourcing trend are also increasing their collaborations.
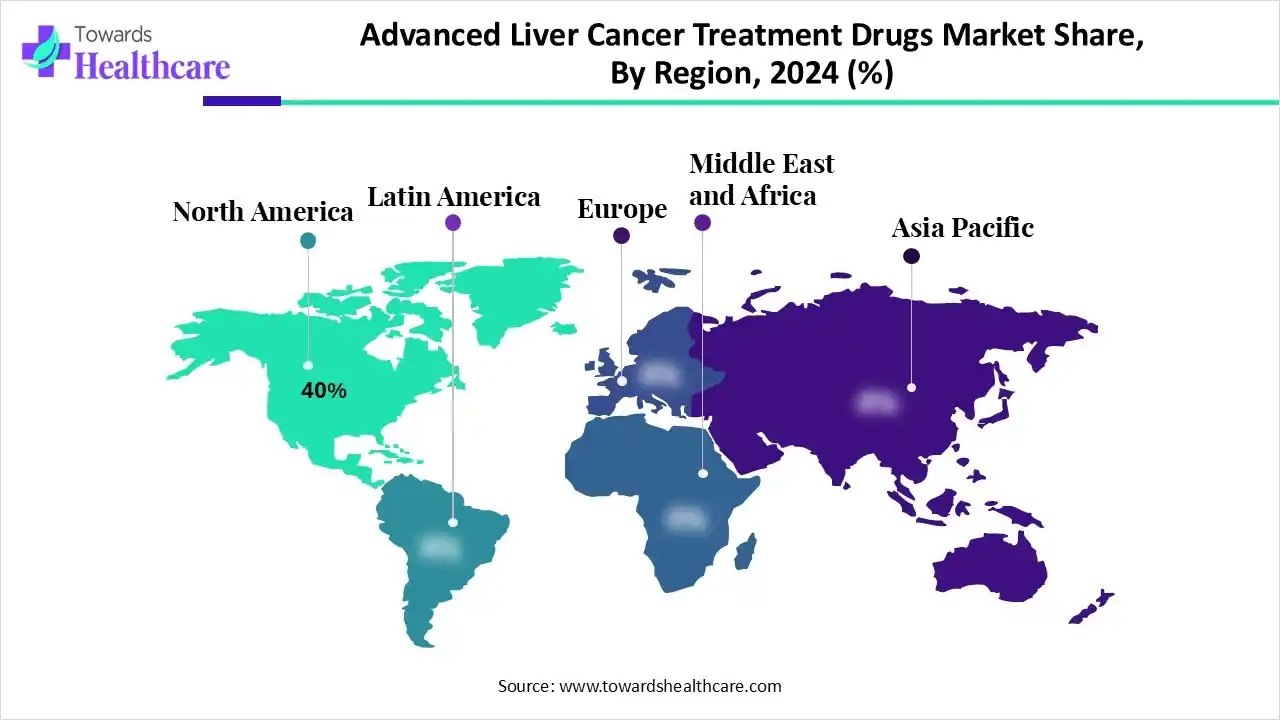
North America dominated the advanced liver cancer treatment drugs market with 40% in 2024, due to established oncology infrastructure and high per-patient spend. At the same the growth in the R&D and clinical trials also increased the innovation in the advanced liver cancer treatment drugs. Moreover, early adoption of novel therapies supported by the healthcare investments also increased their use, which contributed to the market growth.
The presence of advanced healthcare systems in Canada is driving the advanced liver cancer treatment drugs market. The companies are developing various treatments and diagnostic approaches for advanced liver cancer to deal with their growing burdens, which in turn are enhancing their clinical trials. Additionally, the private and government investments and funding are further encouraging innovations.
For instance,
Asia Pacific is expected to host the fastest-growing advanced liver cancer treatment drugs market during the forecast period, driven by increasing incidence of liver cancer and expanding access to targeted and immunotherapy drugs. At the same time, the expanding healthcare and increasing awareness are increasing the demand and the use of advanced liver cancer treatment drugs. This, in turn, is also increasing their R&D, promoting the market growth.
The growing incidence of liver cancer due to lifestyle changes and a large population is enhancing the advanced liver cancer treatment drugs market in India. Moreover, the growing awareness through government campaigns is increasing the use of targeted therapies and immunotherapies. The industries are also developing new treatment options and generic drugs, which are supported by the government initiatives.
For instance,
Europe is expected to grow significantly in the advanced liver cancer treatment drugs market during the forecast period, due to the presence of a strong healthcare sector, which is increasing access and affordability of these drugs. The fast regulatory approvals are encouraging innovations to deal with the growing cancer burden. The industries and institutions are also developing new treatment approaches along with the use of advanced technologies, enhancing the market growth.
The increasing use of alcohol and growing obesity are increasing the liver cancer rates in the UK, driving the advanced liver cancer treatment drugs market. The healthcare sector is increasingly adopting advanced treatment options, which are supported by the government, enhancing their affordability, accessibility, and innovations. The institutes and industries are also collaborating to advance the development of novel therapies.
For instance,
In the UK, liver cancer is the 18th most common cancer in women and has one of the lowest five-year survival rates, with only 13% of people surviving for 5 years or more. There are approximately 6,000 new cases and 6,000 deaths from liver cancer each year. Statistics for 2024 and 2025 are not yet finalized, but the trend shows an increase in cases in Europe, and survival rates vary significantly by stage, with approximately 50% of stage 1 patients surviving for four or more years and only 5% of stage 4 patients.
The R&D of advanced liver cancer treatment drugs focuses on the development of more effective combination therapies by combining immunotherapies with targeted agents and leveraging advanced technologies like AI and cell-based approaches to provide precision medicine and overcome tumor resistance.
Key Players: AstraZeneca, Bristol-Myers Squibb, Eisai Co., Ltd., Bayer AG, Exelixis, Inc., Merck & Co., Inc., Eli Lilly and Company, Roche.
The clinical trials and regulatory approvals of advanced liver cancer treatment drugs involve demonstrating overall survival (OS) or other key endpoints, such as objective response rate and progression-free survival, along with their safety profile and effectiveness.
Key Players: AstraZeneca, Bristol-Myers Squibb, Eisai Co., Ltd., Bayer AG, Exelixis, Inc., Merck & Co., Inc., Roche, Eli Lilly and Company.
The formulation and final dosage preparation of advanced liver cancer treatment drugs include the development of oral capsules or intravenous (IV) infusions, which can be swallowed or administered by diluting the concentrated solution from vials.
Key Players: Bristol-Myers Squibb, AstraZeneca, Eisai Co., Ltd., Bayer AG, Exelixis, Inc., Roche, Eli Lilly and Company, Merck & Co., Inc.
The packaging and serialization of advanced liver cancer treatment drugs focus on the use of sterile vials for IV infusion, bottles, and child-resistant blister packs for oral capsules with a unique 2D barcode on each unit, ensuring traceability and avoidance of counterfeiting.
Key Players: Bristol-Myers Squibb, AstraZeneca, Eisai Co., Ltd., Bayer AG, Exelixis, Inc., Roche, Eli Lilly and Company, Merck & Co., Inc.
Disease education, financial assistance programs from manufacturers, and non-profit, psychological, and emotional counseling are provided in the patient support and services in the advanced liver cancer treatment drugs market.
Key Players: AstraZeneca, Bristol-Myers Squibb, Eisai Co., Ltd., Bayer AG, Exelixis, Inc., Merck & Co., Inc., Roche, Eli Lilly and Company.
By Drug Class/Mechanism of Action
By Treatment Modality
By End User/Customer Type
By Region
January 2026
January 2026
January 2026
January 2026