November 2025

The global lateral flow assays market size was valued at US$ 12.76 billion in 2025 and is projected to grow to US$ 14.23 billion in 2026. Forecasts suggest it will reach approximately US$ 27.40 billion by 2035, registering a CAGR of 7.94% during the period.
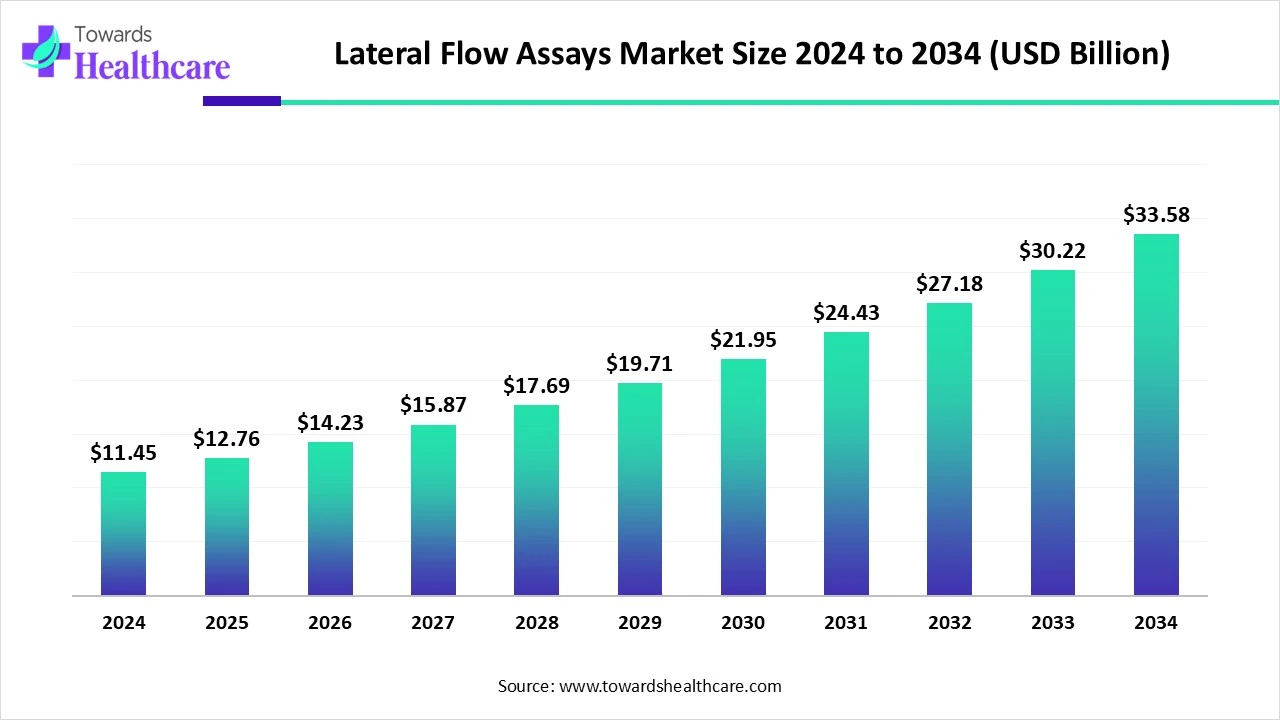
The demand for lateral flow assays is increasing for the rapid, early, and accurate detection of various diseases, fertility testing, drug abuse screening, food safety, etc. This, in turn, is increasing their innovations by integrating with AI. A new set of rules and guidelines is also being introduced by the government to tackle the rising diseases and enhance food safety. This is increasing the development, collaborations, and launches of different types of these tools. Moreover, their demand in different regions is increasing. Thus, this promotes the market growth.
| Table | Scope |
| Market Size in 2025 | USD 12.76 Billion |
| Projected Market Size in 2035 | USD 27.40 Billion |
| CAGR (2026 - 2035) | 7.94% |
| Leading Region | North America Share 37% |
| Market Segmentation | By Product Type, By Technique, By Application, By Sample Type, By End User, By Region |
| Top Key Players | Abbott Laboratories, F. Hoffmann-La Roche Ltd., BD (Becton, Dickinson and Company), QuidelOrtho Corporation, Siemens Healthineers, Bio-Rad Laboratories, Inc., Thermo Fisher Scientific, Inc., Danaher Corporation, Merck KGaA, bioMérieux SA, OraSure Technologies, Inc., Hologic, Inc., Chembio Diagnostics, Inc., Innova Medical Group, ACON Laboratories, Inc., Trinity Biotech plc, SD Biosensor, Inc., Access Bio, Inc., Ellume Limited, Abingdon Health plc |
The lateral flow assays (LFA) market covers rapid, point-of-care diagnostic devices that detect target analytes (antigens, antibodies, nucleic acids, biomarkers) in a sample, typically blood, urine, saliva, or swab, through capillary action on a nitrocellulose membrane. These tests are widely used in infectious disease diagnostics, pregnancy/fertility testing, cardiac marker detection, drug abuse screening, and veterinary diagnostics. Growth is driven by demand for decentralized, low-cost testing, technological advancements (quantitative LFAs, smartphone integration), rising prevalence of chronic and infectious diseases, and sustained post-COVID awareness for at-home and rapid diagnostics.
Increasing government initiatives: To combat the growing diseases, various guidelines and rules are being introduced by the government. Moreover, to improve the health outcomes, new screening programs are also being conducted. This, in turn, is enhancing the detection of diseases and food safety testing.
For instance,
The use of AI in lateral flow assays is increasing to enhance the accuracy of the tools. With the use of AI, the LFA results can be analyzed and scanned using a smartphone camera. This enhances the remote diagnosis. It also helps in providing real-time disease surveillance, as well as can be used for monitoring chronic diseases. They are also being used for the selection of antibodies for the LFA. They can analyze the performance of LFA as well as predict fast negative results.
Growing Point-of-Care Testing Demand
To deal with the growing diseases, the demand for point-of-care testing is increasing. This is increasing the use of the lateral flow assay. These tools offer fast results and can be used across various settings, such as home, ambulances, etc., enhancing the convenience of the patients. At the same time, they are being used for the detection of biomarkers for the management of chronic diseases. This is increasing their innovations for remote patient monitoring. Thus, this drives the lateral flow assays market growth.
Lack of Sensitivity and Specificity
The sensitivity and specificity of these lateral flow assays are considered to be low. This decreases the accuracy of the tools. This, in turn, can lead to false negatives are false positives. Thus, this is increasing the regulatory complexities and increasing the use of molecular diagnostics (PCR). This restrains the use of LFA.
Rising Development of Multiplex and Eco-Friendly LFAs
The demand for the use of multiplex detection is increasing in the healthcare sector. This, in turn, is driving the development of the same for the detection of different diseases. Therefore, innovation for the detection of cancer, cardiac, or other biomarkers is increasing for complex diagnostics. At the same time, the growing environmental concerns are increasing the use of biodegradable and non-toxic materials in LFA. Moreover, new saliva-based testing re being developed due to non-invasiveness. Hence, this is promoting the lateral flow assays market growth.
For instance,
By product type, the kits & reagents segment led the market with approximately 82% share in 2024. The demand for kits and reagents increased due to their recurring use. Moreover, complete test kits for various applications were also provided. This contributed to the market growth.
By product type, the readers/analyzers segment is expected to show the fastest growth rate at a notable CAGR during the predicted time. Their use is increasing due to their portability and quantitative results. At the same time, they are being used with digital platforms. Thus, accurate results and interpretation are increasing their use.
By technique type, the sandwich assays segment held the dominating share of approximately 46% in the market in 2024. They showed improved sensitivity. Thus, they were used in the detection of larger molecules with high specificity. They were also used in the detection of other infectious diseases.
By technique type, the multiplex detection segment is expected to show the highest growth during the predicted time. It offers simultaneous detection of multiple analytes. Moreover, it shows enhanced efficiency and is affordable. Thus, their use in the detection of infectious and chronic diseases is increasing.
By application type, the infectious disease testing segment led the market with approximately 39% share in 2024. The growing incidence of infectious diseases increased the demand for LFA kits. They were used for the detection of respiratory infections (COVID-19, influenza), HIV, malaria, dengue, etc. Thus, this enhanced the market growth.
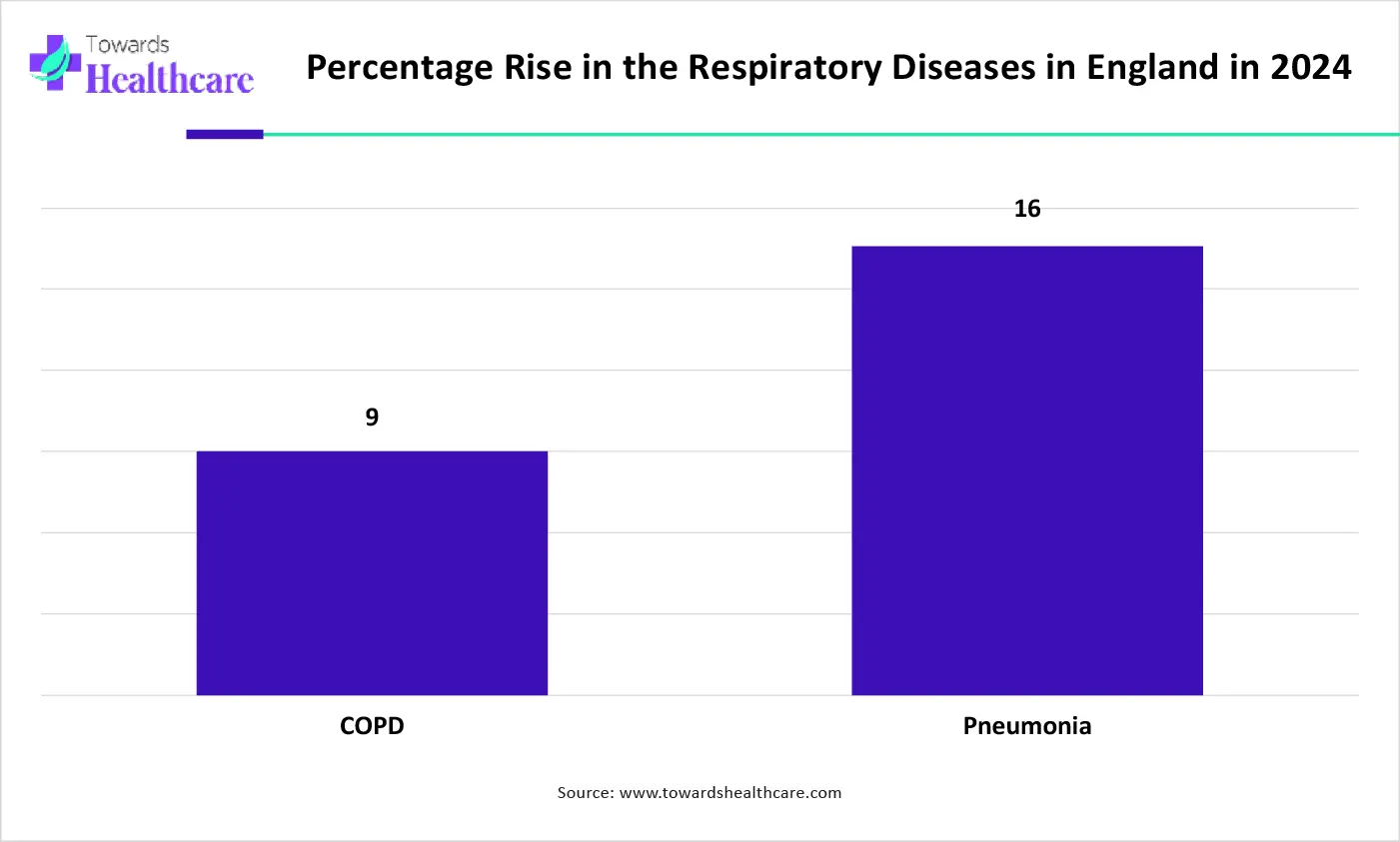
The graph represents the rise in the percentage of emergency admissions due to respiratory infections in England in 2024. It indicates that there will be a rise in cases of respiratory infections. Hence, it increases the demand for the lateral flow assay for its effective detection and management. Thus, this in turn will ultimately promote the market growth.
By application type, the cardiac marker testing segment is expected to show the fastest growth rate during the upcoming years. For the early detection of cardiac disease, the use is increasing. Moreover, the growing demand for rapid MI diagnosis (troponin, CK-MB) is also contributing to the same.
By sample type, the blood/serum/plasma segment held the largest share of approximately 42% in the market in 2024. Different types of analytes were easily detected through these sample types. This, in turn, provided accurate results. Thus, they were widely used in hospitals and labs for various applications.
By sample type, the oral fluid/saliva segment is expected to show the highest growth during the upcoming years. They are preferred due to their non-invasive sampling procedures. This is increasing their use in infectious diseases and DOA tests. Thus, they are also being used for home testing.
By end user, the clinical/diagnostic laboratories segment dominated the global market with approximately 38% share in 2024. The use of LFA increased in these laboratories due to higher volumes of patients. Different types of diseases were detected with its use. Moreover, they were also used for fertility and drug testing. Thus, this promoted the market growth.
By end user, the home care/OTC segment is expected to show the fastest growth rate during the forthcoming years. The LFA kits are being used for COVID self-tests, pregnancy kits, and chronic disease monitoring. Moreover, the OTC availability is increasing its adoption rates. Thus, this is enhancing the patient's convenience and comfort.
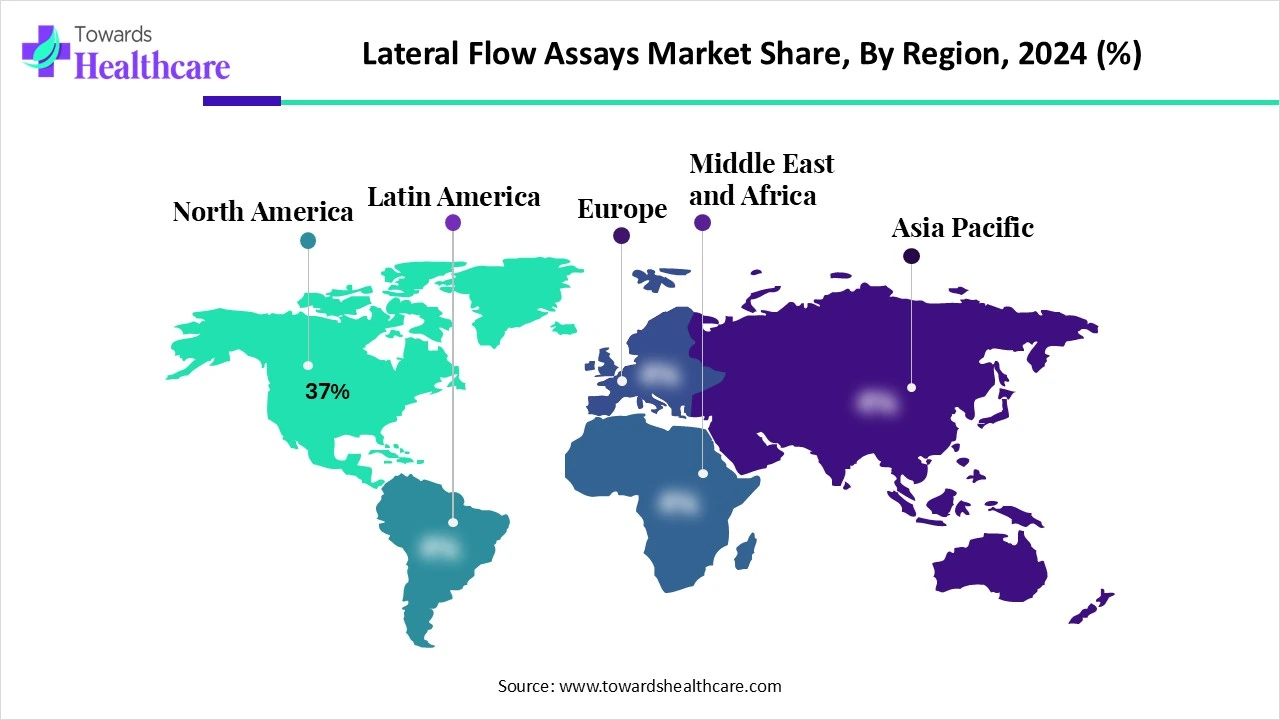
North America dominated the lateral flow assays market share by 37% in 2024. The healthcare sector in North America is well-developed, which has increased the use of the lateral flow assay. They were used for the rapid detection of infectious and chronic diseases. Thus, this contributed to the market growth.
The U.S. consists of well-developed industries. These industries are developing various types of lateral flow assays. At the same time, the use in food testing as well as veterinary diagnostics is increasing. Thus, these developments are supported by the regulatory bodies and the government and are available at various OTC channels.
To deal with the growing infectious disease in Canada, the use of LFA is increasing. At the same time, the growing public health screening programs are increasing their demand. This, in turn, is increasing their development for home care and clinical applications. These are supported by the healthcare investments.
Asia Pacific is expected to host the fastest-growing lateral flow assays market during the forecast period. Asia Pacific is facing a rise in the incidence of infectious diseases. This is increasing the demand for LFA. Thus, this enhances the market growth.
Due to the presence of large populations in China, the risk of diseases increases. Thus, this drives the development of new LFA tools. The industries are utilizing advanced technologies to enhance their sensitivity and specificity. Moreover, the growing OTC retail diagnostics are also contributing to the same.
The growing government-led screening programs are increasing awareness within the population. This, in turn, is increasing the research and development for formulating new LFA tools. Furthermore, the increasing healthcare access is increasing their adoption rates. The government initiatives are also encouraging their adoption.
Europe is expected to grow significantly in the lateral flow assays market during the forecast period. The presence of the advanced health care sector is increasing the investments for the development of new LFA tools. At the same time, growing home testing is increasing the demand for LFA. Thus, this is promoting the market growth.
The industries as well as institutions in Germany are focusing on the development of advanced LFA kits. This, in turn, is increasing the interest in their research and development for new diagnostic kits. Thus, various investments and funding are being provided by the government.
The rising incidences of communicable diseases are increasing the demand for the LFA. At the same time, these tools are also being used for food and environmental testing. Thus, this is enhancing their development and innovations. Moreover, new initiatives by the government are accelerating their adoption.
In June 2025, after announcing the collaboration between Fujirebio and Stanford Medicine, the CEO and president of Fluxus, Inc. (wholly-owned subsidiary of Fujirebio), Dr. Peter Wagner, stated that, by merging the world-renowned research of Stanford with global IVD expertise of Fujirebio and ultrasensitive detection systems of Fluxus, they will take a step toward their mission to improve public health globally. Moreover, they were pleased to work with the prestigious infectious disease experts of Stanford University.
By Product Type
By Technique
By Sample Type
By End User
By Region
November 2025
November 2025
December 2025
November 2025