January 2026

The lung cancer trial market size was valued at US$ 4.34 billion in 2025 and is projected to grow to 4.70 billion in 2026. Forecasts suggest it will reach approximately US$ 9.76 billion by 2035, registering a CAGR of 8.45% during the period.
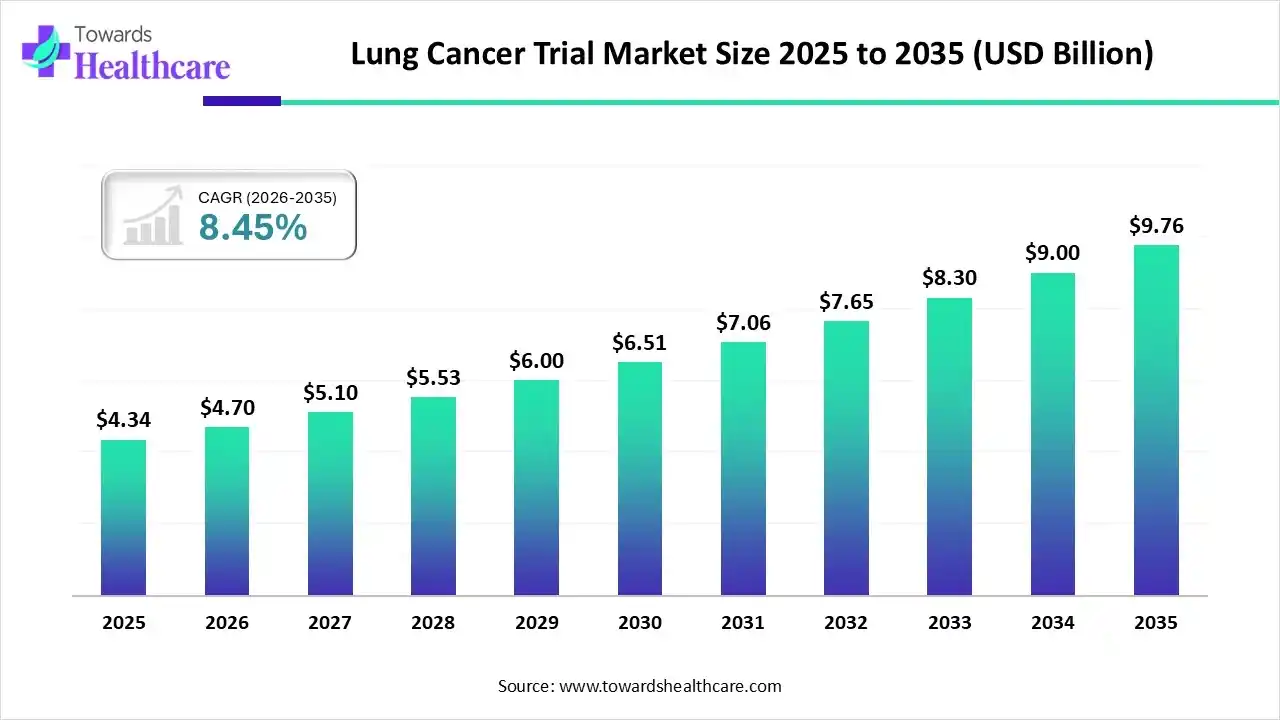
The lung cancer trial market is expanding rapidly due to increasing cases of lung cancer worldwide and the development of personalized treatment. North America is dominant in the market, as high awareness and strong healthcare infrastructure drive the growth of the market.
Furthermore, Asia Pacific is expected to be the fastest-growing due to the low cost of clinical trials and increasing government programmes driving the market growth. Increasing partnership between government institutions and pharmaceutical companies, accelerating clinical trials for lung cancer.
| Key Elements | Scope |
| Market Size in 2026 | USD 4.70 Billion |
| Projected Market Size in 2035 | USD 9.76 Billion |
| CAGR (2026 - 2035) | 8.45% |
| Leading Region | North America |
| Market Segmentation | By Cancer Type, By Therapy Type, By Phase, By Sponsor Type, By Region |
| Top Key Players | AstraZeneca, BoehringerIngelheim, Bristol-Myers Squibb Company, Eli Lilly and Company, Hoffmann-La Roche, Merck & Co, Pfizer Inc., Allergan Inc., TevaPharmaceutical Industries Ltd., AbbVie, Inc., Johnson & Johnson, Amgen Inc., Novartis AG |
The lung cancer trial market is growing rapidly because this trial involves studying novel combinations of immunotherapies with chemotherapy or other medications to treat lung cancer. Lung cancer trials involve how new treatments can be developed and how existing treatments can be enhanced. Clinical trials of lung cancer play a significant role in medical care. Clinical trial providers offer healthcare and more frequent health inspections as part of treatment. This study aims to assess the safety, feasibility, and therapeutic effect of administering a short, quick course of high-dose radiation therapy. Patients with lung cancer should consider novel types of targeted therapies, immunotherapies, and chemotherapies that are available through clinical trials.
Integration of AI in the lung cancer trial drives the growth of the market as AI-driven technology has huge potential to transform early detection and improve patient outcomes. Additionally, further research and integration of AI systems in clinical practice are needed to ensure their safety, reliability, and extensive adoption. This is due to the constant partnership and developments in the medical and AI communities. The current application of AI enables better management and categorization of lung cancer clinical trials, growing trial diversity, and representativeness. AI rapidly analyzes CT scans to identify signs in the lungs and assess who is at risk of developing lung cancer in the upcoming period. This innovation permits earlier treatment and diagnosis.
Increasing Burden of Lung Cancer
The incidence of lung cancer differs globally primarily due to varying risk factors, including smoking rates, environmental pollution, and dietary practices. Exposure to harmful substances, such as air pollution, radon, asbestos, uranium, diesel exhaust, silica, and coal products, heightens the need for early cancer screenings. Additionally, inadequate healthcare conditions and difficulties in implementing tobacco control policies contribute to the expansion of the lung cancer trial market.
Challenges in Computer Vision
Traditional clinical trials have long faced challenges related to startup time, recruitment, accrual, expenses, and the requirement to identify specific subpopulations of patients. These issues often lead to annoyingly slow results, which limit the growth of lung cancer.
Increasing Demand for Personalized Medicine
The rising demand for personalized medicine, which customizes treatments based on an individual’s genetic makeup, plays a vital role in enhancing survival rates and quality of life. In the realm of lung cancer theranostics, precision medicine represents an innovative approach that merges prevention, diagnosis, and treatment tailored to the unique characteristics of individual patients or specific populations. This strategy necessitates the gathering and analysis of genomic, transcriptomic, proteomic, and metabolomic data alongside clinical features of lung cancer patients. Precision medicine serves as a contemporary framework for disease prevention, diagnosis, and treatment, accounting for patients' variances, therefore fueling the need for genotype-specific clinical trials in lung cancer and expanding the opportunities within the lung cancer trial market.
By cancer type, the non-small cell lung cancer (NSCLC) segment dominated and is expected to fastest growing over the forecast period in the lung cancer trial market in 2024, as non-small cell lung cancer is a type of cancer that forms in the lung tissues. There are various types of non-small cell lung cancer. Smoking is a significant risk factor for non-small cell lung cancer. Major signs and symptoms of non-small cell lung cancer include shortness of breath and coughing. NSCLC accounts for around 85% of lung cancers.
By therapy type, the immunotherapy segment is dominant and expected to fastest growing in the lung cancer trial market, as the major development in immunotherapy for lung cancer has been made in immune checkpoint inhibitors. Immune checkpoints are molecules that work on the immune cells, which start or stop an immune response. Immunotherapy is the most promising novel treatment for lung cancer, as it supports the body's immune system to destroy and locate cancerous cells in the lungs. Immunotherapy improves the capacity of the immune system to recognise and eliminate malignant cells, and if traditional therapies fail, the doctor could then suggest immunotherapy for the treatment of lung cancer.
By phase, the phase III segment is dominant in the lung cancer trial market, as investigators evaluate and discover novel treatment strategies that result in an expressive survival advantage for patients with lung cancer is continuously ongoing. The phase III trial proved an improvement in the overall survival rate. Phase III trials goal to capture a wider representation of the population that benefits from the specific drug. In the phase III trials, investigators compare the performance of the drug to other treatment options or a placebo.
For Instance,
The phase II segment is expected to be the fastest-growing in the market due to larger numbers of patients getting a specific drug treatment in Phase II studies than in Phase I studies, and there is a huge chance to detect and compile adverse effect data. These trials offer vital information on the potential risks and efficacy of a drug, supporting researchers in determining whether it has the strength to become a feasible treatment option. In the phase II clinical trials, investigators aim to regulate the drug's effectiveness in treating particular medical conditions.
By sponsor type, the pharmaceutical & biotech companies segment is dominant and expected to fastest-growing in the lung cancer trial market, as clinical trials enhance health care services through increasing the treatment standards. It leads to novel treatments, which support the patients to live longer and to live a life with minimum disability or pain. Clinical trials are significant for drug development and upgrading of human health, and clinical study results are unpredictable. In the pharmaceutical and biotech companies, researchers have tested similar drugs and formulated hypotheses, but they never know the effects of a particular medication or treatment without conducting studies. Clinical trials are used to determine whether novel treatments or medicines developed in the laboratory or by using animal models are effective or safe, or whether any recently developed analytical test will work properly or not.
North America dominated the market in 2024 as the North American healthcare system is considered as ideal healthcare in the world; it provides expedient access to a highly subspecialized network of doctors who work at the forefront of utilizing and developing novel, modern procedures and medications. The North American healthcare system has capitalized on noteworthy resources in pharmaceutical development and medical research, which has led to ground-breaking discoveries and enhanced treatments in a variety of fields, including lung cancer. The presence of a huge cancer center and growing specialized lung cancer programs fast-tracks the demand for lung cancer trials.
For Instance,
The increasing incidence of lung cancer in the United States drives the demand for the market. For Instance, according to the American Cancer Society, in 2025, 2,041,910 new cancer cases and 618,120 cancer deaths are expected to occur in the United States. Tobacco smoking is considered the leading cause of death globally, largely due to the increased risk of lung cancer. Lung cancer is the second most common cancer among men and women in the United States, which contributes to the growth of the market.
In Canada, there is rising evidence that oncology clinical trials units, also growing spending in cancer research, drive the growth of the lung cancer trial market. The Government of Canada is providing funding for pediatric cancer research that leads to better results and healthier, longer lives for young cancer patients. In 2024, it is projected that 32,100 people will be diagnosed with lung cancer in Canada. Lung cancer is expected to continue as the common cause of cancer death, accounting for 23.5% of all cancer deaths, which will cause growth of the market.
The Asia Pacific region is projected to experience the fastest growth in the lung cancer trial market during the forecast period, due to the high prevalence of smoking in the Asia Pacific region, and environmental factors are involved in the surge in lung cancer diagnoses. Air pollution is the fourth-highest risk factor for lung cancer in the Asia Pacific. More than four million people die in the region each year because of long-term exposure to outdoor air pollution.
The increasing incidence and mortality rates of lung cancer, the important cause of cancer death in China, have significantly increased in recent years, leading to an increasing demand for novel treatment and trials in China. China has presented numerous policies and strategies, particularly to address the burden of lung cancer. Enlarged collaboration is required in the different areas in China to offer standardized treatment and relaxing care to patients with lung cancer, which contributes to the growth of the market.
The Indian government has realised various initiatives to make cancer care more affordable and accessible, predominantly for economically weak patients. These programs help to lessen the financial burden of lung cancer treatment, allowing patients to receive effective and timely care. There are some initiatives, such as Ayushman Bharat - Pradhan Mantri Jan Arogya Yojana (AB PM-JAY), National Cancer Control Programme (NCCP), Health Minister's Cancer Patient Fund (HMCPF), and Aarogyasri Scheme, which contribute to the growth of the market.
Europe is expected to grow significantly in the lung cancer trial market during the forecast period, as Europe has an excessively high cancer incidence and mortality burden, given that the continent has one-fifth of the global cancer cases. Huge growth in advanced hospitals, CROs, and research centers in the region supports the large-scale, multi-site trials; these factors all contribute to the growth of the market.
Germany has become a worldwide leader in cancer treatment, renovating many oncological diseases from terminal to manageable conditions, which drives the growth of the market. In Germany, clinical cancer research improves the health results of lung cancer patients, which drives the market.
In June 2025, Ugur Sahin, M.D., CEO and Co-Founder of BioNTech. Stated, “We believe BNT327 has the strength to become an opening immuno-oncology backbone, moving beyond single-mechanism checkpoint inhibitors and growing into multiple solid-tumor indications. Our partnership with BMS, a ground-breaking leader in immuno-oncology, goal to accelerate and largely expand BNT327’s development to completely realize its potential.” (Source - BMS)
By Cancer Type
By Therapy Type
By Phase
By Sponsor Type
By Region
January 2026
January 2026
December 2025
December 2025