January 2026

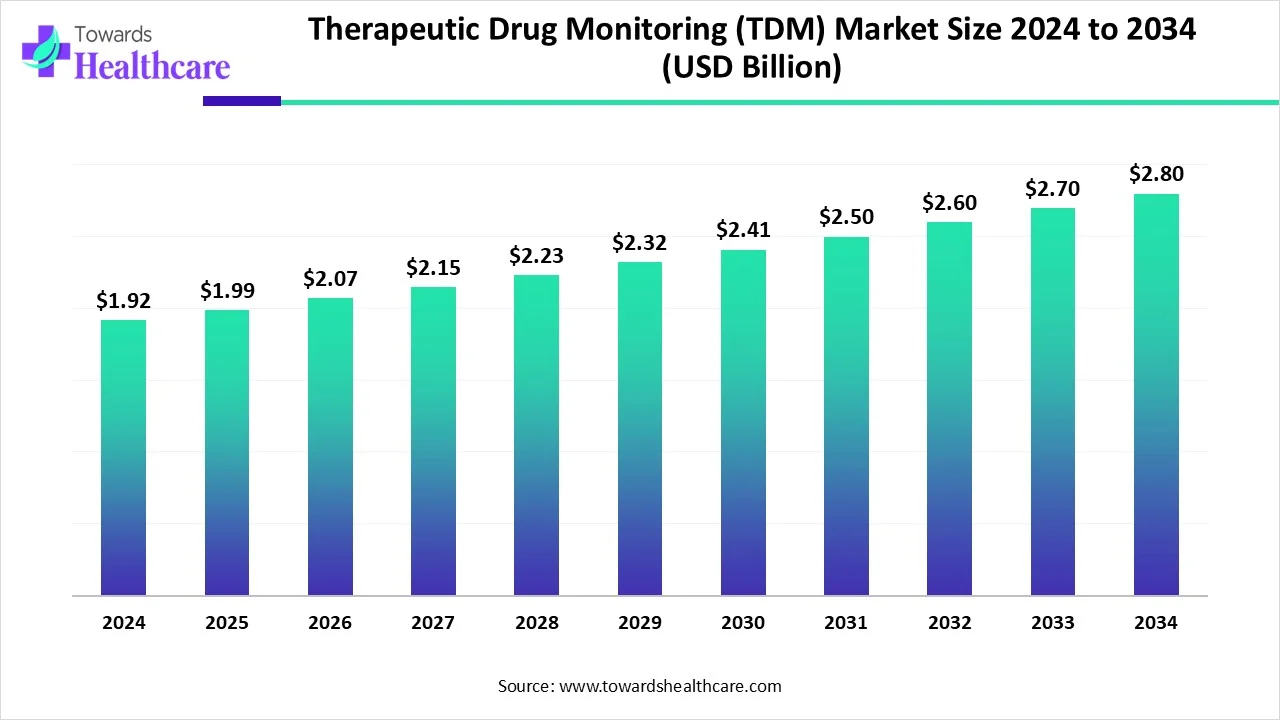
The global therapeutic drug monitoring (TDM) market size is calculated at USD 1.92 in 2024, grew to USD 1.99 billion in 2025, and is projected to reach around USD 2.8 billion by 2034. The market is expanding at a CAGR of 3.84% between 2025 and 2034.
The global therapeutic drug monitoring (TDM) market is experiencing major growth, driven by several factors such as increasing chronic diseases, along with the raised focus on the adoption of personalized medicines, and novel technologies. In the upcoming years, various opportunities are emerging, such as a number of advancements in analytical techniques like immunoassays, chromatography-mass spectrometry have raised accuracy, sensitivity, and throughput of drug monitoring, boosting broad adoption in clinical settings.
| Metric | Details |
| Market Size in 2025 | USD 1.99 Billion |
| Projected Market Size in 2034 | USD 2.8 Billion |
| CAGR (2025 - 2034) | 3.84% |
| Leading Region | North America |
| Market Segmentation | By Product, By Drug Class, By End Use, By Region |
| Top Key Players | Abbott, ALPCO, Beckman Coulter, Inc, Bio-Rad Laboratories, Inc, Thermo Fisher Scientific Inc., Chromsystems Instruments & Chemicals GmbH, F. Hoffmann-La Roche Ltd., Randox laboratories Ltd, Siemens Healthineers AG., biomérieux, Inc. |
Therapeutic drug monitoring (TDM) is a testing that estimates the amount of definite medicines in blood. Also, it helps in checking how much amount of medicine is safe and efficacious. This TDM has been experiencing immense growth due to rising cases of chronic diseases, advancements in personalized medicine, and technological innovations in analytical techniques. This market has a critical role in improving drug dosages, ensuring efficacy, and reducing adverse effects, particularly for drugs with a narrow therapeutic range. Moreover, the growing adoption of companion diagnostics technologies, which assists in anticipating a patient's response to a specific drug, is ultimately involved in the growth of the respective market.
In 2025, AI is playing a major role in several areas, including therapeutic drug monitoring, where it provides more personalized and effective drug administration. Along with this, its algorithms can analyze a huge amount of patient data, such as genetic information, medical history, and lifestyle factors, to estimate individual responses to drugs, determine possible drug-drug interactions, and anticipate adverse drug reactions. This permits highly precise dosing, improved treatment plans, and minimized risks of adverse events. Additionally, AI is applied to monitor drug levels in real-time and maintain dosages accordingly, developing closed-loop systems for personalized drug delivery.
Growing Chronic Disease Cases and Demand for Personalized Medicine
Nowadays, around the globe, different chronic disease instances are rising, including cardiovascular, kidney, and liver diseases, which require long-term medication management. This demands that TDM ensure drug efficacy and reduced toxicity. Moreover, based on the individual patient characteristics, metabolism, and drug responses, TDM plays a vital role in customising drug dosages, resulting in highly efficacious and safer treatment. Besides this, major advancements in various analytical techniques such as immunoassays and chromatography-mass spectrometry have raised accuracy, sensitivity, and throughput of drug monitoring, boosting broad adoption in clinical settings.
High Expenditure and the Need for Well-Trained Personnel
The arising challenges in the therapeutic drug monitoring market, including the implementation of TDM, require costly equipment such as chromatography and mass spectrometry, which is a significant obstacle for smaller institutions. Also, for TDM, proper facilities and well-qualified personnel are required, which may be limiting in some settings.
Accelerating Breakthroughs in Immunoassays and Various Technologies
As immunoassays have a crucial role in TDM, they enable the utilization of specific binding of antibodies to target drugs, giving accurate and sensitive results. Moreover, recent breakthroughs in various technologies such as high-performance liquid chromatography (HPLC), mass spectrometry, and point-of-care testing devices provide highly precise, faster, and robust monitoring of drug levels in patients. Ultimately, these breakthrough in immunoassays and other technologies supports in development of new opportunities in research and development in numerous industries, leading to the growth of the therapeutic drug monitoring (TDM) market.
The consumables segment dominated the market by capturing the largest revenue share of the therapeutic drug monitoring (TDM) market in 2024. This segment includes reagents, kits, blood collection tubes, syringes, and calibrators, which are widely used in drug monitoring, particularly with the rising adoption of advanced immunoassay and chromatography techniques. The dominance of this segment is due to the rising chronic disease cases, with growing awareness of TDM in personalized medicine is boosting the application of consumables.
By product, the equipment segment is expected to grow at a significant CAGR in the upcoming years. This segment is fueled by a number of advancements in analytical technologies and accelerating demand for high-throughput, automated systems. In TDM, highly used equipment includes immunoassay analyzers, chromatography and MS detectors, and clinical chemistry analyzers, which enable more accurate and efficient results during the process.
The antiarrhythmic segment led the global therapeutic drug monitoring (TDM) market in 2024. TDM has a major role in this drug class because of its narrow therapeutic index and potential for serious side effects, like proarrhythmia. Several factors are impacting drug levels and therapeutic response, including active metabolites and protein binding, requiring TDM to enhance dosing and reduce risks.
By drug class, the immunosuppressants segment is expected to grow fastest over the projected period. The therapeutic drug monitoring market includes these drugs experiencing a vital growth, as the increasing number of obesity instances due to lifestyle changes, which fuels demand for the expansion of the drugs used to treat rheumatoid arthritis, multiple sclerosis, and psoriasis. Also, the growing autoimmune disease and organ transplant cases demand immunosuppressants to reduce the immune system’s attack on healthy cells and prevent rejection in organ transplants.
By end use, the hospital segment led the market with a dominating revenue share of the therapeutic drug monitoring (TDM) market in 2024. As a growing number of organ transplant procedures and the rising importance of precision medicine and treatments are fueling the growth of the hospital segment. Hospitals enabling all the required facilities, advanced techniques, and medications are driving the market expansion.
The diagnostics segment is expected to grow at the fastest CAGR during the forecast period. The segment is propelled by rising diagnostics centers and enhanced quality, and well-established infrastructure of treatment centers. They are broadly focusing on development, testing, and introducing diagnostic tools to the market, which can be used anywhere for health applications.
North America held the major revenue share of the market, due to increasing chronic issues, including cardiovascular, neurological concerns, diabetes, and cancer, which demand TDM for improving treatment and reducing adverse effects. Also, this region is highly emphasizing on development of tailored drug dosages to fulfill each patient's needs, ensuring potential therapeutic outcomes and minimizing the risk of toxicity.
In the US, the escalating novel technologies, including immunoassay technologies (ELISA, chemiluminescence, etc.) and chromatography-mass spectrometry (chromatography-MS), enable optimized accuracy and efficiency of TDM are critically impacting market growth. Also, the robust government regulations relate to safe medication practices, especially for drugs with narrow therapeutic ranges, boosting the adoption of TDM.
Canada has been experiencing a crucial growth in the respective, fueled by the growing well-developed infrastructure in the healthcare systems, along with innovations in technologies, providing accurate and efficient outcomes in TDM.
In Asia Pacific, expected to grow at the fastest CAGR in the studied years. The market is driven by rising chronic disease cases, which need more precise and robust medications, fueling the TDM. Also, raised emphasis on investments in healthcare over the ASAP, which provides access the advanced diagnostic services and technologies, is supporting the market growth.
China has been exploring the wide range of applications of numerous technologies, including immunoassays and liquid chromatography (including HPLC and LC-MS) in TDM. As well as, they are possessing more hospitals, particularly larger ones, which provide all the services, and are propelling the market growth.
India has been adopting widespread technological advancements along with the accelerating investments in research and development, and infrastructure, which are enhancing the access and quality of TDM services.
Europe is experiencing significant growth of the respective market, with contributing factors such as rising demand for personalized medicines and growing awareness about the advantages of the same, which has boosted the adoption of TDM in recent years. Also, they are highly emphasizing the healthcare infrastructure and innovative technologies.
TDM is playing a vital role in Germany by contributing to rising prevalences, raised adoption of personalized medicines, and growing investment in novel technologies.
In the UK, the rising breakthroughs in drug monitoring tools, strong government support, and accelerating investments in healthcare infrastructure, R&D, and innovative technologies are impacting the expansion of the market.
In April 2025, Bruker, a leading high-throughput ‘chrom-free’ ClinDART triple-quad MS platform, announced its investment in RECIPE, a leading provider of Mass Spectrometry-based Diagnostic Assay Kits for Therapeutic Drug Monitoring. Jeffrey Zonderman, Senior Vice President of Bruker Applied Mass Spectrometry, commented that this investment aligns with the planned vision for LC-TQ-MS assays, accelerated with novel chrom-free assays based on proprietary DART® technology which resulting in clinical research and regulated TDM markets. (Source- Businesswire)
By Product
By Drug Class
By End Use
By Region
January 2026
January 2026
January 2026
January 2026