February 2026

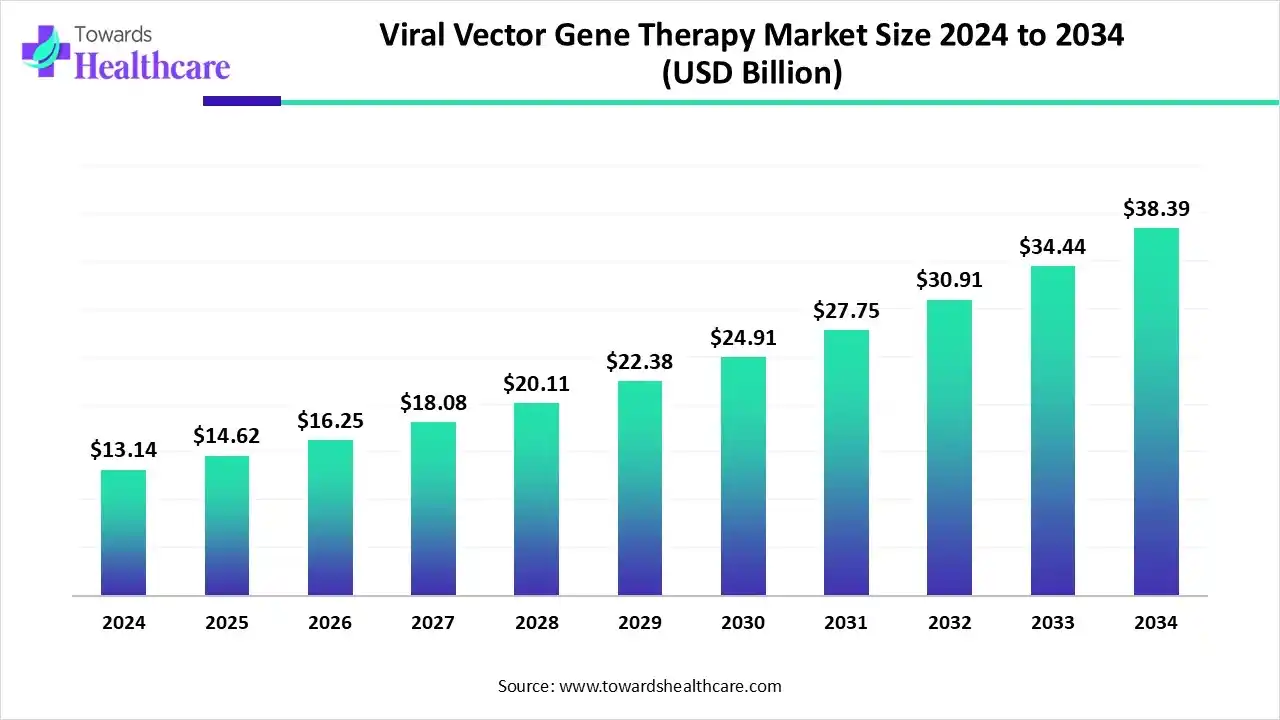
The viral vector gene therapy market size reached US$ 13.14 billion in 2024 and is anticipate to increase to US$ 14.62 billion in 2025. By 2034, the market is forecasted to achieve a value of around US$ 38.39 billion, growing at a CAGR of 11.23%.
The viral vector gene therapy market is expanding rapidly due to increasing demand for precise and long-lasting treatments for genetic, rare, and chronic diseases. Technological advancements in vector design and delivery systems have improved the safety and effectiveness of these therapies. Additionally, rising investments in research, favorable regulatory support, and growing success in clinical trials are encouraging pharmaceutical companies to develop novel viral vector-based treatments. These factors, combined with increasing awareness and diagnosis of genetic disorders, are driving the growth of this market.
Viral vector gene therapy is a form of treatment that uses genetically engineered viruses as carriers to deliver healthy genes into a patient's cells, aiming to correct or replace faulty genes responsible for specific diseases. The viral vector gene therapy market is growing due to increasing demand for advanced treatments for genetic and rare disorders. Improved vector technologies have enhanced the safety and efficiency of gene delivery, making therapies more effective. Rising investment in research, growing success in clinical trials, and supportive regulatory approvals are also driving development. Additionally, greater awareness and diagnosis of genetic conditions, along with breakthroughs in personalized medicine, are fueling the expansion of this innovative treatment market.
For Instance,
AI is transforming the market by accelerating drug discovery, optimizing vector design, and improving manufacturing efficiency. It helps identify ideal gene targets, predict vector behavior, and streamline clinical trial processes through advanced data analysis. AI also enhances quality control and reduces development time, making therapies more cost-effective and accessible. These capabilities are driving innovation and faster commercialization, supporting the growing demand for precise and personalized gene-based treatments.
Rising Prevalence of Genetic and Rare Disorders
The increasing number of genetic and rare disease cases is pushing the need for more effective and targeted therapies. Viral vector gene therapy offers a promising approach by correcting faulty genes at the source, providing lasting benefits that conventional treatment often can’t achieve. With advances in diagnostics and awareness, more patients are being identified, increasing demand for innovative therapies. This trend is fueling research, development, and investment in the viral vector gene therapy market.
For Instance,
High Cost and Complexity of Manufacturing
The complex and costly manufacturing process of viral vectors poses a major barrier to growth in the gene therapy market. It involves sophisticated equipment, highly skilled personnel, and strict regulatory standards, making large-scale production difficult. These factors contribute to long development timelines and limited availability of therapies. As a result, treatment prices remain high, reducing affordability and access for many patients, and creating challenges for broader market expansion and adoption across healthcare systems.
Advanced Therapies
Advanced therapies offer a promising future for the viral vector gene therapy market by enabling more efficient and targeted treatment approaches. Emerging innovations, such as synthetic vectors, tissue-specific delivery systems, and improved payload capacity, are enhancing the therapeutic potential of gene therapy. These breakthroughs not only expand the range of treatable conditions but also improve treatment outcomes. As research progresses, these advancements are expected to make gene therapies more accessible and sustainable for a larger patient population.
For Instance,
The adeno-associated virus segment lead the viral vector gene therapy market in 2024 because of its strong clinical success and flexibility in treating a range of genetic diseases. Their ability to carry therapeutic genes with minimal side effects and deliver them precisely to targeted tissues has made them a preferred choice for developers. Additionally, ongoing innovations and regulatory approvals using the AAV platform have further strengthened their role as a dominant presence.
For Instance,
The lentivirus segment is projected to grow at the fastest rate in the viral vector gene therapy market due to its ability to integrate genetic material into both dividing and non-dividing cells, making it highly effective for long-term gene expression. It is especially valuable in therapies involving stem cells and hematological disorders. The growing focus on treating complex genetic diseases and successful clinical outcomes using lentiviral vectors is driving increased research investment and accelerated expansion in the market.
The gene therapy segment led the viral vector gene therapy market in 2024 and is expected to grow at the fastest CAGR during the forecast period due to its ability to provide long-term or curative treatments for genetic disorders. Rising demand for innovative therapies, especially for conditions with limited or no treatment for genetic disorders. Rising demand for innovative therapies, especially for conditions with limited or no treatment options, has accelerated their development. Continuous breakthroughs in viral vector technologies and increasing support from regulatory bodies have further encouraged market growth.
The pharmaceutical and biotechnology companies segment dominated the market because of its robust capabilities in innovation, technology integration, and regulatory navigation. These companies are at the forefront of developing novel gene therapies and advancing vector production techniques. Their strong global presence, strategic alliances, and access to funding enable them to drive clinical progress and bring therapies to market efficiency, making them a key player in pushing the viral vector gene therapy market.
The Contract Research Organizations (CROs) & Contract Manufacturing Organizations (CMOs) segment is experiencing accelerated growth in the viral vector gene therapy market as pharmaceutical and biotech firms increasingly outsource complex development and production tasks. These organizations offer advanced technical capabilities, regulatory expertise, and scalable infrastructure, which help reduce operational costs and speed up clinical and commercial timelines. As gene therapies become more advanced and widely adopted, CROs and CMOs are playing a vital role in supporting innovation, ensuring quality, and meeting the rising global demand efficiently.
North America dominated the market in 2024 owing to its concentration of cutting-edge research institutions, skilled workforce, and early adoption of advanced therapeutic technologies. The region's emphasis on personalized medicine, combined with frequent clinical trials and government support for gene-based innovations, fuels ongoing progress. Additionally, collaborations between biotech startups and major pharmaceutical companies further enhance development capabilities, making North America a key hub for breakthroughs in viral vector gene therapy.
The U.S. market is growing due to heavy investment from both private and public sectors in biotech R&D, increasing FDA approvals of novel therapies, and a mature regulatory system that supports advanced clinical development. The country’s leading infrastructure, including specialized manufacturing facilities and numerous partnerships among academia, startups, and major pharma, is accelerating vector production and trial progress. These strengths are driving rapid market growth across the U.S.
For instance,
Canada’s market is growing due to increasing focus on advanced therapies for genetic and rare diseases, along with supportive government initiatives in biotechnology. The country has witnessed a rise in clinical trials and partnerships between academic institutions and biotech firms, fostering innovation in gene delivery technologies. Additionally, improvements in manufacturing infrastructure and rising patient awareness are encouraging the adoption of gene therapies, making Canada a key player in this evolving healthcare segment.
Asia-Pacific is anticipated to grow rapidly in the market during the forecast period due to rising collaborations between regional biotech firms and global pharmaceutical companies, along with growing government support for innovative treatment approaches. The region's large and diverse population presents significant opportunities for clinical research. Moreover, increased focus on rare disease diagnosis and treatment, along with improving regulatory frameworks, is encouraging the adoption and development of gene therapies, boosting market growth across emerging economies in the region.
China's market is expanding rapidly due to significant biotech investments and strong government initiatives aimed at boosting domestic vector production. The country benefits from a growing pool of patients with genetic disorders and enhanced clinical research efforts, including increased trial activity. Improved infrastructure, such as specialized manufacturing facilities, and supportive national policies also foster innovation, making China a key emerging hub for viral vector-based therapies.
India's market is expanding due to a combination of increased investment in biotech infrastructure, supportive government initiatives, and a growing focus on rare and genetic disorders. Companies like Bharat Biotech and emerging CDMOs are enhancing domestic manufacturing of viral vectors such as AAV and lentivirus. Additionally, India’s large patient population and improved clinical trial capabilities are attracting global collaborations, positioning the country as a rising hub for gene therapy research, development, and production.
Europe is driving the market forward by enhancing manufacturing capacity, increasing research funding, and fostering innovation through supportive regulatory initiatives. In 2024, several European companies, such as Exothera and RDBiotech, expanded their viral vector production facilities to meet rising demand. Additionally, the European Union’s Horizon Europe program and updated regulations from the European Pharmacopoeia have improved development and quality standards for gene therapies, helping streamline clinical translation and commercial readiness across the region.
The UK market is expanding due to strong government support, advanced R&D infrastructure, and a growing number of GMP-certified manufacturing facilities. As of 2024, the UK has significantly increased its capacity for producing AAV and lentiviral vectors, which are crucial for gene therapy. This growth is also driven by collaborations between biotech firms and research institutions, allowing faster development and commercialization of therapies. These advancements position the UK as a key hub for gene therapy innovation in Europe.
Germany’s market is witnessing growth due to increasing research collaborations, rising demand for advanced gene-based treatments, and strong government and academic support. Leading biotech companies are partnering with research institutions to develop innovative therapies, especially in areas like rare genetic diseases and cancer. The country’s well-established regulatory framework, robust healthcare infrastructure, and skilled workforce further support the development and commercialization of viral vector-based gene therapies, making Germany a key player in the European biotech landscape.
In 2024, NewBiologix launched its Xcell™ rAAV Production and Analytics Platform to improve the efficiency, quality, and scalability of viral vector production for gene and cell therapy. Built on the proprietary Xcell™ Eng-HEK293 cell line, the platform allows parallel screening of multiple rAAV gene candidates and offers complete data reporting for regulatory needs. CEO Igor Fisch stated that this platform was developed to reduce production risks and timelines, helping gene therapies advance more efficiently into clinical development. (Source: Busniesswire)
By Vector Type
By Application
By End-User
By Region
February 2026
February 2026
February 2026
January 2026