APAC Central Lab Market Size, Key Players with Insights and Dynamics
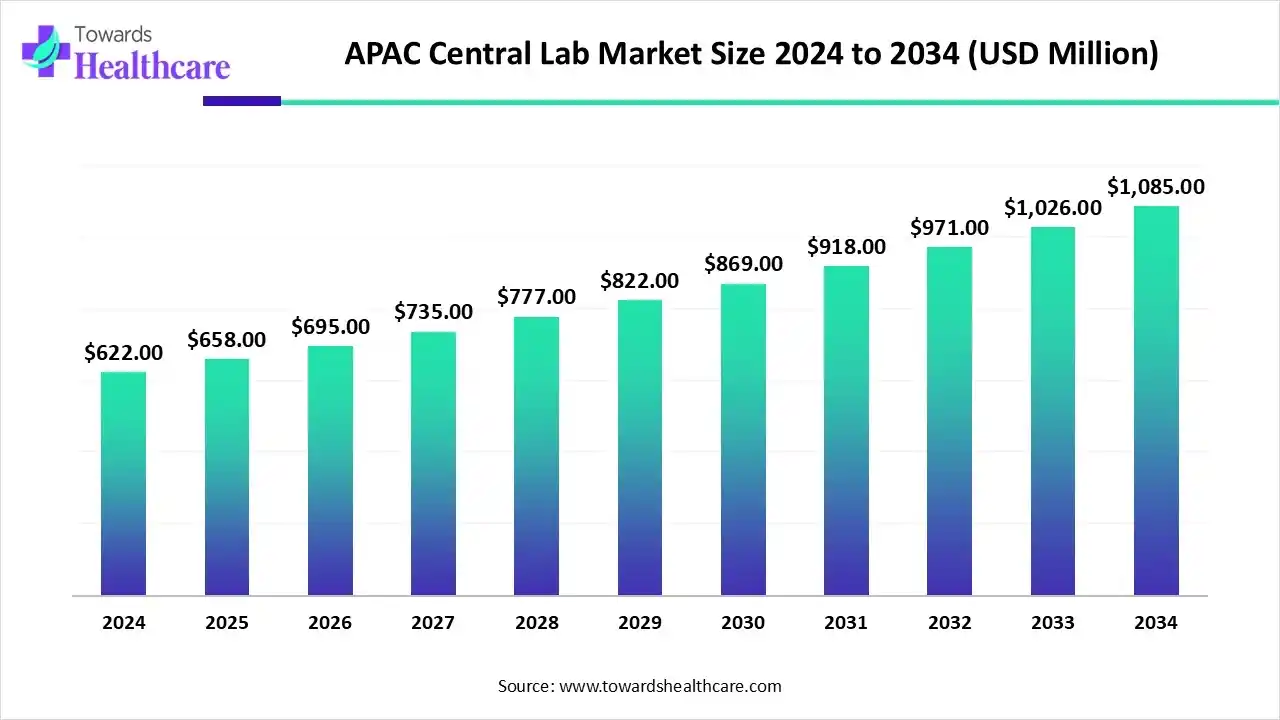
The APAC central lab market size was estimated at US$ 622 million in 2024, projected to increase to US$ 658 million in 2025 and reach US$ 1085 million by 2034, showing a healthy CAGR of 5.72% across the forecast years.
The APAC central lab market is expanding rapidly due to increasing adoption of advanced diagnostic technologies, rising awareness of precision medicine, and growing demand for efficient clinical trial support. The region benefits from cost-effective laboratory services, rising pharmaceutical R&D, and expanding collaborations between local labs and global CROs. Additionally, technological innovations, enhanced sample management capabilities, and increasing regulatory compliance initiatives are driving the market’s growth, making APAC a significant contributor to the global central lab sector.
Key Takeaways
- APAC central lab market to crossed USD 622 million by 2024.
- Market projected at USD 1085 million by 2034.
- CAGR of 5.72% expected in between 2025 to 2034.
- The global central lab market is set to grow from USD 3.46 billion in 2024 to USD 6.04 billion by 2034 at a 5.71% CAGR, driven by R&D and clinical trial demand.
- China dominated the APAC central lab market with a revenue share of approximately 50% in 2024.
- By service type, the genetic services segment held the largest market share of approximately 30% in 2024.
- By service type, the biomarker services segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By laboratory type, the hospital-based laboratories segment led the APAC central lab market with the largest revenue of approximately 45% share in 2024.
- By laboratory type, the independent and reference laboratories segment is expected to grow at the fastest CAGR in the market during the forecast period.
- By application, the preclinical and clinical trial-related services segment held the highest market share of approximately 40% in 2024.
- By application, the cell and gene therapy-related services segment is expected to grow at the fastest CAGR in the market during the forecast period.
Key Indicators and Highlights
| Table |
Scope |
| Market Size in 2025 |
USD 658 Million |
| Projected Market Size in 2034 |
USD 1085 Million |
| CAGR (2025 - 2034) |
5.72% |
| Market Segmentation |
By Service Type, By Laboratory Type, By Application, By Region
|
| Top Key Players |
Charles River Laboratories, Medpace, Frontage Laboratories, SGS S.A., Eurofins Scientific, REPROCELL Inc., ACM Global Laboratories, LabConnect, Cerba Research, Synevo Central Labs, Versiti, Cenetron, Ampersand Capital Partners, Pacific Biomarkers, Cirion BioPharma Research
|
What is APAC Central Lab?
The APAC central lab market is growing due to rising clinical trials, expanding pharmaceutical R&D, and increasing adoption of advanced laboratory and diagnostic technologies across the region. The market comprises centralized laboratory facilities providing comprehensive services to support clinical trials, research, and diagnostic testing across. These labs offer services such as genetic testing, biomarker analysis, microbiology, anatomic pathology, and specimen management to pharmaceutical, biotechnology, and healthcare organizations.
Driven by rising healthcare investments, increasing clinical trial activities, and the adoption of precision diagnostics, the APAC central lab market is witnessing significant growth and technological advancement, enabling improved efficiency, data accuracy, and regulatory compliance across the region.
APAC Central Lab Market Outlook
- Industry Growth Overview- The market is growing steadily, driven by increasing clinical trials, expanding pharmaceutical research, adoption of advanced lab technologies, rising outsourcing, and government support for healthcare infrastructure and diagnostics.
- Global Expansion- The market seeks global growth by strengthening cross-border partnerships, increasing capacity for multinational clinical trials, integrating innovative diagnostic solutions, and positioning the region as a reliable hub for high-quality laboratory services.
- Major Investors- Key investors in the APAC central lab market include regional biotech firms, multinational pharmaceutical companies, healthcare technology providers, and strategic investment funds supporting expansion of laboratory networks, innovative testing services, and clinical trial capabilities.
How Can AI Affect the Market?
AI is transforming the APAC central lab market by enabling intelligent sample management, automated reporting, and enhanced pattern recognition for complex datasets. It facilitates faster clinical trial analyses, improves operational efficiency, and reduces costs. Additionally, AI supports advanced predictive modeling, multi-omics data interpretation, and quality control, helping labs meet regulatory standards while accelerating research and drug development. This integration strengthens lab capabilities, enhances service scalability, and positions APAC as a competitive hub for innovative central laboratory solutions.
What are the Key Trends in the APAC Central Lab Market in 2024?
- Expansion of Pharmaceutical and Biotech R&D- Growth in pharmaceutical and biotechnology research in the region fuels demand for specialized laboratory services, biomarker testing, and analytical support.
- In September 2025, LyondellBasell will upgrade its Suzhou Technical Center in China with a new extrusion line and processing workshop, enhancing compounding for complex polypropylene and engineered plastics, and enabling faster, innovative solutions for automotive, electronics, and consumer goods.
- Innovation in Laboratory Technologies- Adoption of advanced testing methods, automation, and high-throughput technologies improves accuracy, efficiency, and scalability of lab services across clinical trials.
- In July 2025, Carbon Clean opened its Global Innovation Centre in Navi Mumbai, India, featuring carbon capture plants and advanced labs. The facility accelerates research and deployment of CycloneCC, its modular, cost-effective carbon capture technology.
Segmental Insights
How does the Genetic Services Segment dominate the APAC Central Lab Market in 2024?
In 2024, the genetic services segment led the market with the revenue shares of approximately 30%, as healthcare providers and research organizations increasingly relied on genetic testing for personalized treatment planning, risk assessment, and disease prevention. The rise of innovative technologies, such as high-throughput sequencing and advanced genomic analysis, along with expanding collaborations between biotech firms and central laboratories, fueled demand.
The biomarker services segment in genetic services is set to grow rapidly as healthcare systems in APAC increasingly adopt data-driven and predictive diagnostic methods. Growing collaborations between biotech firms and research institutions, rising focus on companion diagnostics, and government support for genomic research are fueling demand.
Why Did the Hospital-based Laboratories Segment Dominate the APAC Central Lab Market in 2024?
The hospital-based laboratories segment captured the largest revenue share of approximately 45% in 2024, owing to the growing demand for precision diagnostics and advanced testing within hospital settings. These labs ensure real-time coordination between clinicians and laboratory professionals, enhancing diagnostic accuracy and treatment outcomes.
The independent and reference laboratories segment is projected to expand rapidly as clinical trials become more decentralized and require broader geographic coverage. These labs provide specialized expertise, flexible capacity, and faster turnaround times for diverse testing needs. Growing collaborations with CROs and pharmaceutical companies for complex studies, along with the adoption of AI-driven data analytics and remote sample management systems, are further boosting their efficiency and making them preferred partners for large-scale, multi-site research projects.
How does the Preclinical and Clinical Trial-Related Services Segment Dominate the APAC Central Lab Market?
The preclinical and clinical trial-related services segment dominated the market with the revenue shares of approximately 40% in 2024 as sponsors increasingly outsourced testing to central labs for consistent quality and regulatory compliance. Growing complexity in drug development and personalized medicine has heightened the need for centralized data management, faster turnaround times, and advanced analytical support.
The cell and gene therapy–related services segment is expected to record the fastest growth as clinical research expands into rare diseases and precision medicine. Increasing collaborations between pharmaceutical companies and central labs are driving demand for specialized analytical support and sample management.
Regional Insights
How is China contributing to the Expansion of the APAC Central Lab Market?
China is driving Market growth with the revenue shares of approximately 50%, by emerging as a hub for specialized and high-throughput laboratory services. Increasing private sector investments, expansion of independent and reference laboratories, and integration of digital and automated lab technologies are boosting efficiency. Furthermore, partnerships with international pharmaceutical companies and CROs for multi-site clinical trials, along with growing focus on precision medicine and biomarker research, are strengthening China’s role as a key contributor to the region’s central lab market expansion.
India Market Trends
India is fueling the APAC Central Lab Market’s expansion by emerging as a preferred destination for outsourced laboratory services and multi-site clinical trials. The country’s growing network of independent and hospital-based laboratories, increasing adoption of automated and high-throughput testing technologies, and rising focus on biomarker and genomic research are driving market growth. Strategic collaborations with global pharmaceutical companies and CROs, along with ongoing infrastructure development, further strengthen India’s role in the region’s central lab ecosystem.
Japan Market Trends
Japan’s APAC Central Lab Market is expanding as the country focuses on innovative drug development and regenerative medicine research. Rising investment in laboratory automation, high-throughput testing, and genomic analysis enhances service capabilities. Additionally, Japan’s emphasis on regulatory compliance, collaborations with international pharmaceutical companies, and growing participation in multinational clinical trials are driving demand for centralized lab services, positioning the country as a key contributor to APAC’s overall central laboratory market growth.
Global Central Lab Market Growth
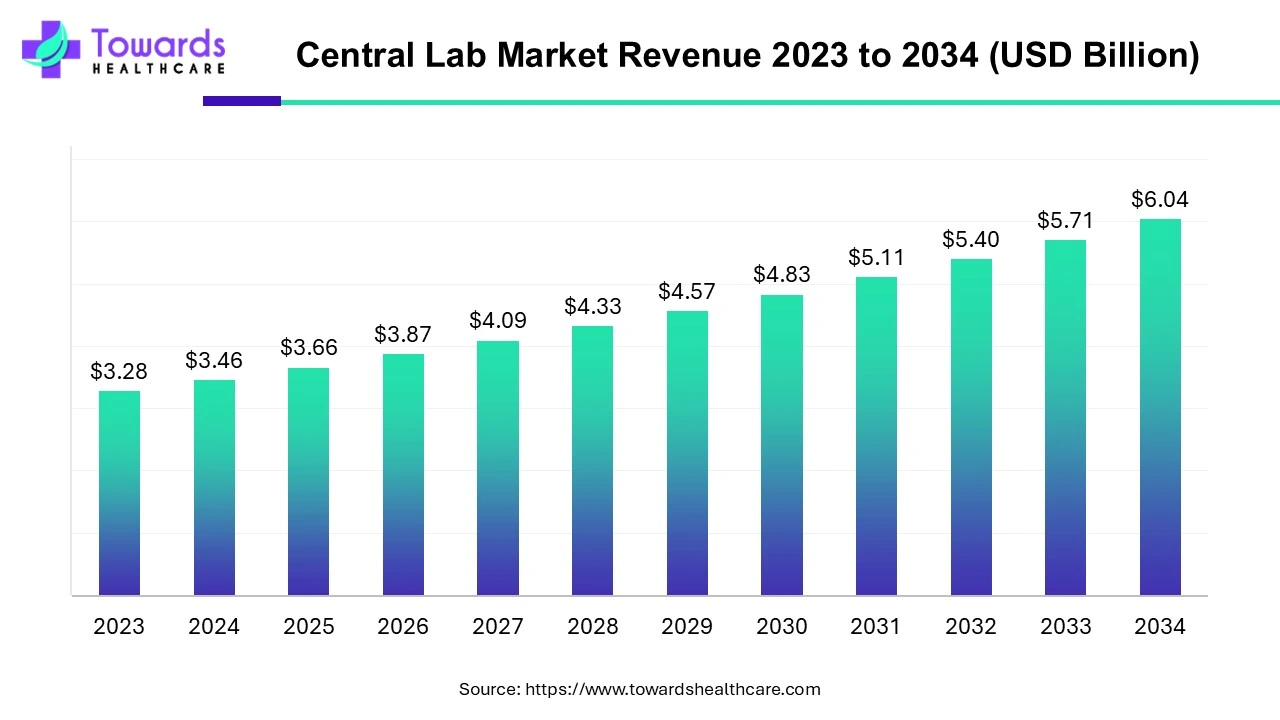
The global central lab market is valued at USD 3.46 billion in 2024 and is projected to reach USD 6.04 billion by 2034, growing at a CAGR of 5.71% from 2024 to 2034, driven by increasing investments in R&D and a growing demand for clinical trials.
APAC Central Lab Market Value Chain Analysis
Clinical Trial
- Clinical trials using an APAC central lab involve sending samples to a centralized lab in the Asia-Pacific region for standardized, high-quality testing.
- This ensures consistent, accurate, and comparable data across all trial sites.
- APAC is becoming a key hub for clinical research due to large patient populations and a growing number of trials.
- Compliance with global standards like GCP makes the region a preferred choice for central lab services in international studies.
Serialization
- Serialization in APAC central labs involves tracking and managing clinical trial materials to prevent counterfeit medicines.
- The process is complex due to diverse national regulations across the region.
- Unlike the standardized rules in the U.S. or EU, APAC requires a flexible, multi-level approach to comply with developed, emerging, and loosely regulated markets.
- Effective serialization ensures safety, compliance, and integrity of clinical trial supplies across the region.
Patient Support and Services
- In APAC, patient support services for central labs focus on clinical trial management and diagnostic testing.
- Services follow a collaborative model involving laboratories, CROs, healthcare providers, and technology platforms.
- The approach emphasizes supporting trials and diagnostics rather than direct-to-patient care.
Top Vendors and their Offerings
- Thermo Fisher Scientific- Thermo Fisher Scientific provides comprehensive laboratory solutions, including analytical testing, central lab services, genomic and molecular diagnostics, sample management, and clinical trial support, enabling pharmaceutical, biotech, and healthcare organizations to advance research efficiently.
- IQVIA- IQVIA provides end-to-end life sciences solutions, including laboratory testing, biomarker analysis, data management, and technology-enabled clinical trial support, helping pharmaceutical and biotech companies accelerate research, ensure compliance, and improve patient outcomes worldwide.
- ICON plc- ICON plc delivers specialized laboratory and clinical trial services, including sample processing, advanced diagnostics, regulatory compliance support, and integrated study management, enabling pharmaceutical and biotech companies to conduct efficient, high-quality global research programs.
- Labcorp- Labcorp delivers end-to-end clinical research solutions, offering specialized testing, pharmacology studies, patient sample logistics, and data management services, helping pharmaceutical and biotech companies optimize drug development and streamline global trial operations.
- Quest Diagnostics- Quest Diagnostics offers specialized laboratory solutions, including high-throughput testing, biomarker analysis, sample management, and clinical research support, enabling pharmaceutical, biotech, and healthcare organizations to accelerate studies and maintain regulatory compliance globally.
Top Companies in the APAC Central Lab Market
Recent Developments in the APAC Central Lab Market
- In March 2025, IQVIA Laboratories launched Site Lab Navigator, a solution designed to automate and optimize lab workflows for clinical trial sponsors and investigator sites. The platform features an e-Requisition system, enabling electronic submission of lab requests and streamlined management of specimens.
- In June 2024, Labcorp introduced Labcorp Global Trial Connect, a suite of central lab solutions designed to accelerate clinical trials. The platform offers services including site enablement, supply management, workflow optimization, and investigator and study support.
Segments Covered in the Report
By Service Type
- Genetic Services
- Genotyping
- Gene Expression Analysis
- Next-Generation Sequencing (NGS)
- Whole Genome/Exome Sequencing
- Biomarker Services
- Pharmacodynamic Biomarker Testing
- Predictive Biomarker Testing
- Diagnostic Biomarker Testing
- Microbiology Services
- Bacterial Testing
- Viral Testing
- Fungal Testing
- Anatomic Pathology/Histology
- Tissue Biopsy Analysis
- Immunohistochemistry (IHC)
- Histopathology
- Specimen Management & Storage
- Biobanking
- Sample Logistics & Tracking
- Cold Chain Storage
- Special Chemistry Services
- Clinical Chemistry
- Immunoassays
- Toxicology Analysis
By Laboratory Type
- Independent and Reference Laboratories
- Hospital-Based Laboratories
- Nursing and Physician Office-Based Laboratories
By Application
- Drug Discovery Related Services
- Target Identification & Validation
- Preclinical Screening
- Lead Optimization
- Drug Development Related Services
- Clinical Trial Sample Analysis
- Pharmacokinetics/Pharmacodynamics
- Biomarker Validation
- Bioanalytical and Lab Chemistry Services
- Chromatography & Spectrometry
- Immunoassays & ELISA
- Toxicology Testing Services
- In Vitro Toxicology
- In Vivo Toxicology
- Cell and Gene Therapy Related Services
- Viral Vector Testing
- Cell Characterization
- Gene Editing Analytics
- Preclinical and Clinical Trial Related Services
- GLP-Compliant Testing
- Data Management & Reporting
- Other Clinical Laboratory Services
- Specialty Diagnostics
- Companion Diagnostics
By Region
- Asia Pacific
- Japan
- China
- India
- South Korea
- Australia
- Thailand



