February 2026

The global human platelet lysate market size is calculated at US$ 587 million in 2025, grew to US$ 677 million in 2026, and is projected to reach around US$ 2416 million by 2035. The market is expanding at a CAGR of 15.19% between 2026 and 2035.
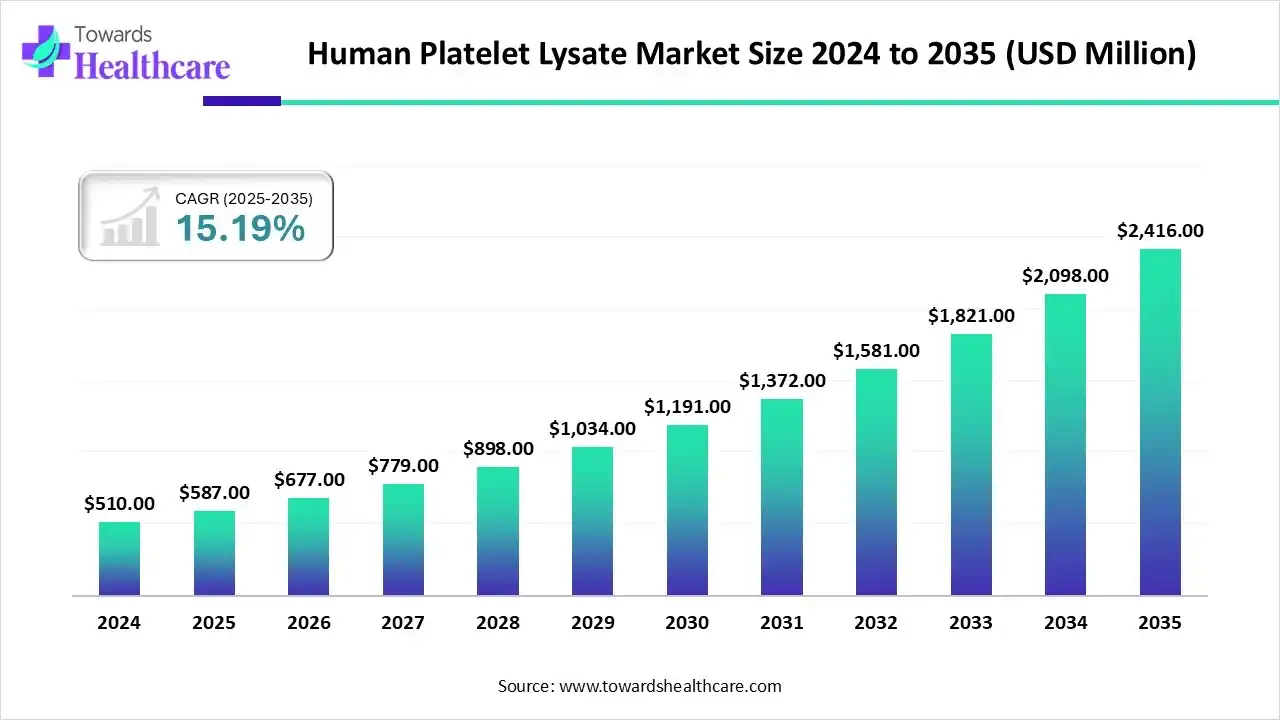
HPL's use in a variety of treatments has increased as a result of the rising demand for cutting-edge therapeutic solutions brought on by the rising prevalence of chronic illnesses. The human platelet lysate market has grown as a result of ongoing research and development in regenerative therapies, which have increased the use of HPL.
| Table | Scope |
| Market Size in 2025 | USD 587 Million |
| Projected Market Size in 2035 | USD 2416 Million |
| CAGR (2026 - 2035) | 15.19% |
| Leading Region | Europe by 41% |
| Market Segmentation | By Type, By Application, By End User, By Source, By Region |
| Top Key Players | Macopharma SA, Compass Biomedical Inc., Merck KGaA (Sigma-Aldrich), Mill Creek Life Sciences LLC, Stem Cell Technologies Inc., Cook Regentec, Biological Industries (a Sartorius company), ZenBio Inc., PL BioScience GmbH, Trinova Biochem GmbH, Thermo Fisher Scientific Inc., Corning Incorporated, EMD Millipore Corporation, AventaCell BioMedical Corp., Celprogen Inc., SeraCare Life Sciences, Innovative Research Inc., Anova IRM GmbH, iBiotech S.r.l., Rees Scientific Corporation |
The human platelet lysate market is the global industry that produces and supplies platelet-derived supplements used as growth media for cell culture, particularly in regenerative medicine, stem cell therapy, and biopharmaceutical research. HPL is obtained from human platelet concentrates through lysis, releasing growth factors and cytokines that support cell proliferation without the use of animal-derived sera like fetal bovine serum (FBS). Its advantages, xeno-free composition, safety, and regulatory acceptance, are driving adoption in clinical-grade cell manufacturing and research applications.
With companies like PL BioScience and Captivate Bio obtaining capital and forming alliances, the human platelet lysate market is a thriving startup ecosystem. These forward-thinking businesses concentrate on providing high-quality, standardized, and reasonably priced HPL solutions to satisfy the particular needs of the cell therapy industry.
Which Type Segment Dominated the Market in 2024?
The pooled human platelet lysate segment accounted for approximately 58% of the human platelet lysate market in 2024 because in 2D and 3D culture systems, pooled human platelet lysate may be more advantageous than fetal bovine serum for the growth of mesenchymal stem cells. Essential platelet-derived growth factors and cytokines can be balanced by pooling individual platelet lysate units into a single HPL batch. The safety of HPL will be further improved by the increasingly used pathogen-reduction technologies.
Pathogen-Inactivated Platelet Lysate
The pathogen-inactivated platelet lysate segment is estimated to grow at the highest CAGR during the predicted timeframe. By inactivating newly discovered pathogens that are not detected by existing screening or testing procedures, pathogen inactivation (PI) technologies aim to improve blood safety. By halting their replication, this method renders a variety of pathogens inactive bacteria, viruses, and protozoa using substances such as psoralen and ultraviolet (UVA) light.
Single Donor Platelet Lysate
The single donor platelet lysate segment is expected to grow significantly in the human platelet lysate market during the forecast period. Reduced donor exposure through Single Donor Platelet Lysate (SDPL) lowers the risk of immunological responses and disease transmission. Because it contains a large number of growth factors, it is primarily used in regenerative medicine, cell therapy, and as a supplement to cell cultures to encourage tissue repair and cell proliferation.
Why Cell Therapy & Regenerative Medicine Dominated the Market in 2024?
The cell therapy & regenerative medicine segment dominated the human platelet lysate market, accounting for approximately 47% of revenue. Because human platelet lysate is rich in growth factors and is being used as a novel therapeutic approach, researchers have shown a great deal of interest in it. In addition to their use in cell therapy, human platelet lysates have the potential to be used for regenerative tissue, including bone regeneration, androgenetic alopecia, tendon regeneration, and nerve repair due to their high concentration of growth factors.
Others
The others segment is estimated to grow at the highest CAGR during the predicted timeframe. Human platelet lysate (HPL) is used as a therapeutic agent and as a cell culture supplement in immunotherapy and regenerative medicine, as well as a source of biomarkers in diagnostics. It is extremely valuable due to its rich composition of cytokines, growth factors, and other bioactive molecules.
Biopharmaceutical Production
The biopharmaceutical production segment is expected to grow significantly in the human platelet lysate market during the forecast period. Platelet-derived bioproducts have been suggested as a possible tool in the field of tissue healing as regenerative medicine drives new solutions and our understanding of the critical role platelets play in this process continues to grow. The use of PRP in sports medicine and orthopaedics has been growing in popularity very quickly.
Which End-User Dominated the Market in 2024?
The biotechnology & pharmaceutical companies segment dominated the human platelet lysate market, accounting for approximately 42% of revenue. HPL is a high-potential product used in various procedures. From research, to diagnostics, biopharmaceutical production, cell therapy, immunotherapy, and so on. With so many usage and applications, HPL becomes a great choice for the biotechnology & pharmaceutical companies to use in clinical research and other aspects of healthcare services.
Hospitals & Clinical Centers
The hospitals & clinical centers segment is estimated to grow at the highest CAGR during the predicted timeframe. Hospitals and clinical centers are key hubs for treating patients; therefore, the products developed by pharma and biopharma companies using HPL are utilized by hospitals and clinics in diagnostics and therapeutics.
Academic & Research Institutes
The academic & research institutes segment is expected to grow significantly in the human platelet lysate market during the forecast period. HPL has been recent studies and has great potential in developing advanced therapeutics. Therefore, various academic & research institutes actively conduct research to study the nature and therapeutic usage of HPL.
Why Expired Platelet Units Became Dominant in the Market in 2024?
The expired platelet units segment accounted for approximately 60% of the human platelet lysate market revenue. According to reports, up to 20% of all platelet units are thrown away because they expire before they can be used. Therefore, after platelets expire, they may be used for purposes other than transfusion. Additionally, using platelet units that have expired poses no ethical issues and does not conflict with patients' need for platelet transfusions. As a result, HPL made from expired platelet units is a practical and affordable substitute for FBS in MSC culture.
Platelet Apheresis Products
The platelet apheresis products segment is estimated to grow at the highest CAGR during the predicted timeframe. An automated device is used to prepare apheresis platelet products from a single donor collection. The ability to produce more kinds of platelet products is a benefit of an apheresis platelet collection system. They can either be suspended in 100% donor plasma, or a platelet additive solution (PAS) can be used to replace a portion of donor plasma, usually about 65%. PAS platelets have the advantage of lowering the frequency of allergic transfusion reactions and transferring less incompatible plasma to recipients who are ABO incompatible.
Fresh Platelet Concentrates
The fresh platelet concentrates segment is expected to grow significantly in the human platelet lysate market during the forecast period. When it comes to aggregation and functionality, fresh platelet concentrates are superior to frozen ones. When applied to tissue regeneration, they provide a high concentration of immune cells and growth factors to encourage quick tissue repair and healing.
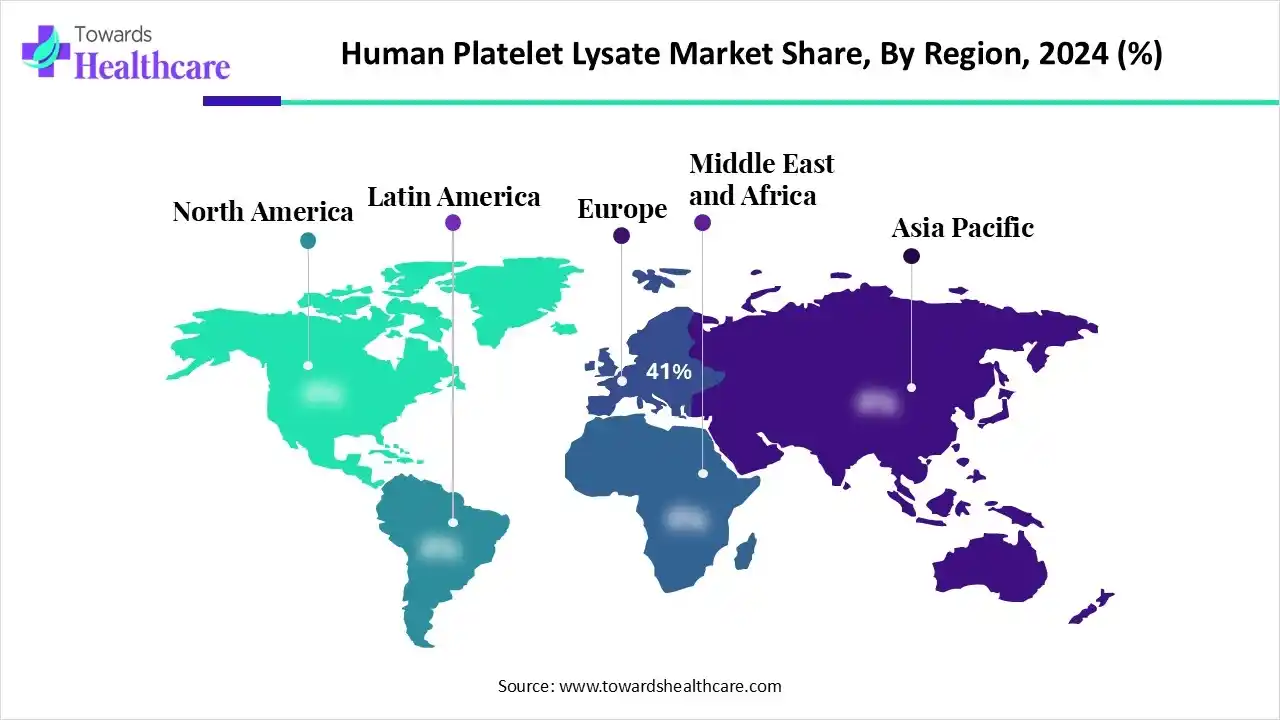
Europe dominated the human platelet lysate market share by 41% in 2024 due to the region's robust biomedical research focus, growing regenerative medicine industry, and favorable regulatory environment. Regulatory agencies and ethical committees oversee the use of HPL in Europe to ensure that clinical and research uses respect established ethical standards and do not endanger the health of patients or donors.
With hubs in Munich, Berlin, Heidelberg, and other places, Germany is home to one of the most vibrant biotech ecosystems in Europe. In addition to the internationally renowned biotech firms like BioNTech, Germany is home to a diverse startup community developing the newest technologies.
Asia Pacific is estimated to host the fastest-growing human platelet lysate market during the forecast period driven by a rise in the prevalence of chronic diseases and rising healthcare costs. Government programs that support regenerative medicine research and improve healthcare infrastructure are important growth drivers. With substantial contributions from both domestic and foreign players, China is by far the largest market in the region.
The potential of HPL to treat a number of chronic illnesses is being researched. More than 70% of the economic disease burden and 88.5% of all deaths in China were caused by chronic illnesses. Chronic illnesses are now the primary cause of avoidable early mortality in humans, which not only raises the financial burden of illness but also has a negative influence on day-to-day functioning and employment, greatly lowering health-related quality of life (QOL).
North America is expected to grow at a significant CAGR in the human platelet lysate market during the forecast period because of the increasing use of human platelet lysate in cell culture, the expansion of human platelet lysate research and development, and the regulatory bodies' supportive backing. The area has a highly developed research infrastructure, and the life sciences and regenerative medicine are both heavily funded.
Two organizations have received cooperative agreements worth up to $1.5 million from the National Institute of Standards and Technology (NIST) of the U.S. Department of Commerce to develop programs and curricula for educating the present and future workforce in regenerative medicine on standards implementation. Each of the two cooperative agreements has an annual renewal option of up to three years and is for $250,000.
South America is expected to grow significantly in the human platelet lysate market during the forecast period. Driven by expanding regenerative medicine research and increasing adoption in cell therapy centers. Clinic industry collaborations are strengthening while funding for biotechnology in South America supports broader growth across the continent.
Brazil’s biotech ecosystem is advancing with specialized cell-culture labs embracing human platelet lysate to replace fetal bovine serum in research applications. Local regulatory agencies and emerging contract-research organizations are creating favourable conditions for suppliers and manufacturers to scale operations in Latin American markets.
The Middle East and Africa are expected to grow at a lucrative CAGR in the human platelet lysate market during the forecast period. In the Middle East and Africa the market is emerging as hospitals upgrade their regenerative medicine platforms and research foundations launch stem-cell studies. Governments in GCC nations and Africa are investing in advanced therapeutic infrastructure and fostering regional manufacturing partnerships for growth.
Gulf Cooperation Council states are actively cultivating biotechnology clusters with investor interest in cell-therapy supplies, including human platelet lysate. Clinical centers, medical-tourism hubs, and domestic manufacturers are positioning to meet demand from regenerative medicine applications and regional research initiatives
Enhancing scalability, improving safety, and creating standardized production processes for advanced therapeutic applications and regenerative medicine are the main goals of research and development in the human platelet lysate market.
Key companies involved are Merck KGaA, Mill Creek Life Sciences, and Compass Biomedical.
Human platelet lysate's safety and effectiveness in cell-based treatments are confirmed by clinical trials. While promoting adherence to GMP guidelines and ethical sourcing practices in both domestic and foreign markets, regulatory bodies expedite the approval process.
Key companies involved are Cook Regentec, Sartorius AG, and Macopharma.
Programs for patient support place a strong emphasis on accessibility, education, and post-therapy monitoring. Clinics and biopharma companies work together with service providers to guarantee safe product use, quality control, and better treatment results.
Key companies involved are STEMCELL Technologies, Corning Incorporated, and BioLife Solutions.
Company Overview
Merck KGaA is a leading German science and technology company with a strong focus on Healthcare, Life Science, and Electronics. The Life Science business, where Human Platelet Lysate (HPL) products are housed (often under the MilliporeSigma brand in North America), provides tools, materials, and services for scientific research and bioprocessing.
Corporate Information:
History and Background:
History: Began as a pharmacy in Darmstadt. Over centuries, it evolved into a global chemical and pharmaceutical group. Its Life Science sector was significantly bolstered by the 2015 acquisition of Sigma-Aldrich, which expanded its portfolio in research and high-tech lab materials, including cell culture media and supplements like HPL.
Key Milestones/Timeline:
Business Segments/Divisions:
Geographic Presence: Operates in over 65 countries; sales are globally diversified, with significant presence in North America and Europe.
Key Offerings:
Key Developments and Strategic Initiatives:
Technological Capabilities/R&D Focus:
Competitive Positioning:
SWOT Analysis:
Recent News and Updates:
Company Overview: STEMCELL Technologies is a global biotechnology company that develops specialized cell culture media, cell separation technologies, instruments, and services for life science research, focusing heavily on stem cells, immunology, and regenerative medicine core application areas for HPL.
Corporate Information:
History and Background:
History: Founded by Dr. Allen Eaves, a leading cancer and stem cell researcher, with a core mission of providing standardized, high-quality, and innovative reagents and tools to advance stem cell research globally. Its expertise in stem cell media formulation naturally led to the development of specialized Human Platelet Lysate products.
Key Milestones/Timeline:
Business Segments/Divisions:
Key Offerings:
End-Use Industries Served
Academic and Research Institutes, Biotechnology Companies (especially cell and gene therapy developers), Biopharma CDMOs.
Key Developments and Strategic Initiatives:
Technological Capabilities/R&D Focus:
Competitive Positioning:
SWOT Analysis:
Recent News and Updates:
| Company | Offerings and Contributions |
| Macopharma SA | Provides GMP-grade platelet lysate, ensuring global supply reliability and standardization for regenerative medicine applications. |
| Compass Biomedical Inc. | Develops xeno-free platelet lysate, advancing safer and reproducible therapeutic cell culture solutions. |
| Mill Creek Life Sciences LLC | Produces PLTMax for scalable serum-free cell manufacturing supporting tissue engineering growth. |
| Cook Regentec | Offers clinical-grade platelet lysate media enhancing regulatory compliance and therapeutic translation. |
| ZenBio Inc. | Supplies high-quality lysate for research use supporting consistent, ethical biotechnology and life sciences applications. |
Read further to see how top players are redefining the Human Platelet Lysate Market: https://www.towardshealthcare.com/companies/human-platelet-lysate-companies
By Type
By Application
By End User
By Source
By Region
February 2026
December 2025
December 2025
November 2025