November 2025

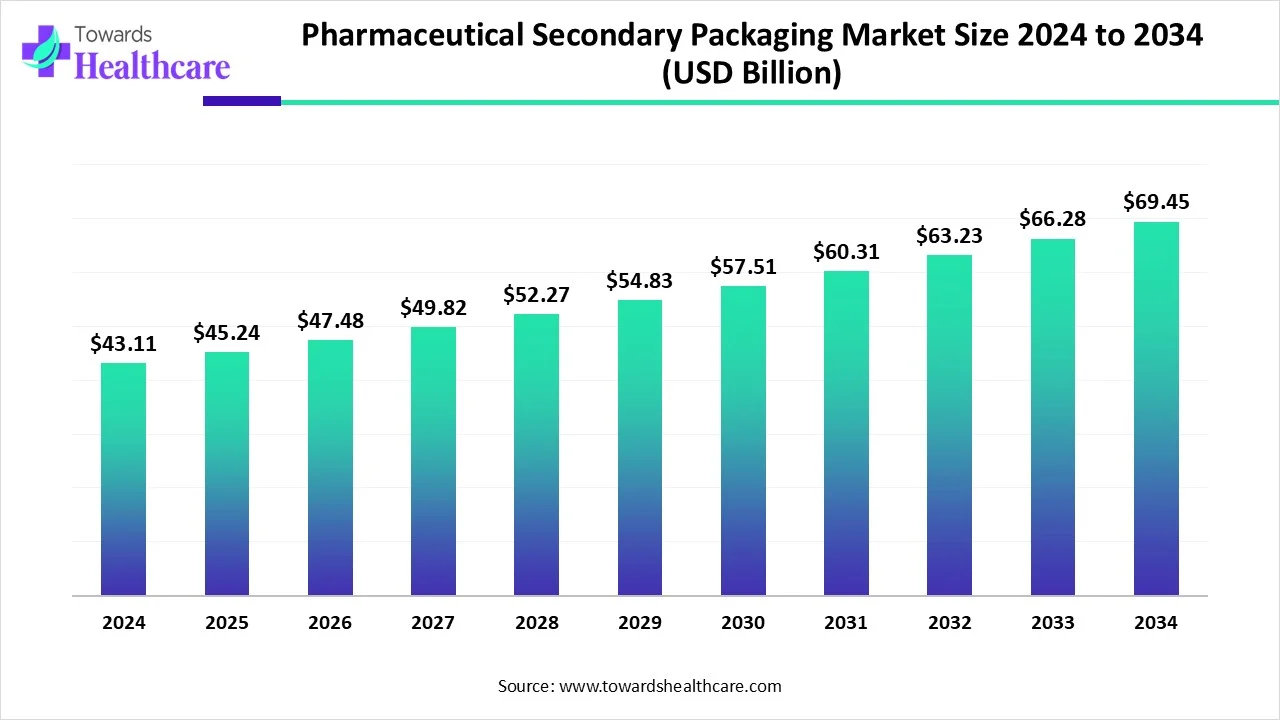
The global pharmaceutical secondary packaging market size is calculated at USD 43.11 in 2024, grew to USD 45.24 billion in 2025, and is projected to reach around USD 69.45 billion by 2034. The market is expanding at a CAGR of 4.94% between 2025 and 2034.
| Metric | Details |
| Market Size in 2025 | USD 45.24 Billion |
| Projected Market Size in 2034 | USD 69.45 Billion |
| CAGR (2025 - 2034) | 4.94% |
| Leading Region | North America |
| Market Segmentation | By Packaging Type, By Material Type, By Application, By End Use, By Region |
| Top Key Players | WestRock, Amcor, Metsä Board, Huhtamaki, Sonoco, Gerresheimer, Berry Global, Schreiner MediPharm, CCL Industries, Körber Pharma Packaging, Graphic Packaging International, Constantia Flexibles |
The secondary packaging adds another layer of information and protection to the primary packaging, which is the container that immediately holds the pharmaceutical item. The integrity, safety, and appropriate distribution of pharmaceutical medicines from producers to end users are all dependent on secondary packaging in the pharmaceutical sector. The effectiveness and safety of pharmaceutical goods are maintained throughout their lifespan by secondary packaging, which makes sure that important regulatory information is present, protects against tampering, and adds an extra layer of protection.
Artificial intelligence (AI), blockchain, and automation might speed up the pharmaceutical packaging process and enhance the traceability of finished units. AI may also be applied to packing processes to detect defects like product contamination and leakage that result in waste and might put people at risk. Deep-learning AI models can offer a more comprehensive analysis than traditional image processing techniques. They can detect and adapt to changing ambient conditions, such as different degrees of light. All things considered, AI might help ensure a more accurate product count, improve quality assurance, and expedite the packing process.
Rising Demand for Anti-Counterfeit Packaging
As concerns about product contamination and pharmaceutical counterfeiting grow, tamper-evident packaging solutions are gaining popularity. The most often used techniques for detecting illicit drug access are shrink bands, blister packs, breakable caps, and sticky closures. The industry is also witnessing developments in smart tamper-proof technology, where embedded NFC (Near Field Communication) chips enable consumers and authorities to instantly verify the authenticity of products. These advancements are expected to lead to a major expansion in the pharmaceutical secondary packaging business.
Strict Regulations
The secondary pharmaceutical packaging market is subject to strict restrictions from both national and international governments and agencies. Because patient safety is the primary priority, strict regulations must be followed by both pharmaceutical medications and final packaged items. Businesses find it challenging to provide unique, high-quality packaging.
Is Sustainable Packaging the Next Thing in the Pharmaceutical Secondary Packaging Market?
The importance of sustainability is growing. Biodegradable or recyclable pharmaceutical packaging materials are becoming more and more popular in the industry. For instance, the use of conventional plastics is being lessened by bioplastics produced from renewable resources like corn starch. Aluminum and glass are also being utilized since they are reusable without degrading in quality.
By packaging type, the folding cartons segment led the pharmaceutical secondary packaging market in 2024. These foldable paper boxes, as their name implies, are made to be easily assembled from a single flat sheet into a three-dimensional package. They are frequently used as secondary packaging and are reasonably priced to ship because they are shipped flat before being assembled, while still offering a high degree of customization.
By packaging type, the blister packs segment is expected to be the fastest-growing in the pharmaceutical secondary packaging market during the forecast period. The packaging of pharmaceuticals has been transformed with the invention of blister packs. Once considered a straightforward safety measure, blister packs have developed into a sophisticated tool for ensuring medication integrity and enhancing patient compliance. Blister packs are becoming more and more popular due in part to a greater understanding of patient requirements, since they offer a practical way to manage drugs, especially for the elderly and those with chronic conditions that require regular treatment.
By material, the paperboard segment held the largest revenue of the pharmaceutical secondary packaging market in 2024. In the pharmaceutical industry, which is moving toward environmentally friendly packaging, paperboard is the most commonly utilized material for secondary packaging. It may be used in packaging, labels, and inserts, and is lightweight and extremely recyclable. Despite the government's continued limitations on the use of plastic, pharmaceutical businesses are increasingly employing paperboard packaging that is biodegradable and FSC-approved in order to promote sustainability without compromising package integrity and compliance.
By material, the plastic segment is expected to achieve the highest CAGR in the pharmaceutical secondary packaging market during the forecast period. Because of their adaptability, plastics have completely changed packaging. Commonly utilized materials include polyvinyl chloride (PVC), high-density polyethylene (HDPE), and polyethylene terephthalate (PET). They are perfect for a variety of items due to their lightweight, indestructible nature, and capacity to be molded into different forms. These goods include dropper bottles, blister packs, and bottles. Biodegradable plastics are another example of plastic technological advancements that support environmental sustainability objectives.
By application, the injectables segment captured the major revenue of the pharmaceutical secondary packaging market in 2024. Because autoinjectors make it easier for people to self-administer medication rather than needing a qualified healthcare practitioner, the market for injectables is expanding. Furthermore, in order to cut waste and overall carbon emissions, there is a growing demand for environmentally friendly, sustainable solutions, such as biodegradable material substitutes. Pharmaceutical packaging has evolved, yet it still has to produce a user-friendly, compliant design without sacrificing patient safety.
By application, the biologics segment is expected to grow rapidly in the pharmaceutical secondary packaging market during the predicted time. Secondary packaging in biologics is primarily used for cold chain support, patient comfort and safety, regulatory compliance, and product protection. The secondary packaging shields biologics against contamination, impact, and environmental exposure. The regulatory compliance ensures that sterilization, labeling, and track-and-trace systems meet international requirements.
By end-use, the pharmaceutical companies segment was dominant in the pharmaceutical secondary packaging market in 2024. To improve their usefulness and safety, package businesses are putting more and more emphasis on incorporating tamper-evident qualities, administration aids, sustainable materials, dispensing systems, and counterfeiting precautions into their products. To improve package sustainability, businesses are primarily concentrating on using post-consumer recycled (PCR) materials and creating packaging from biodegradable materials.
By end-use, the contract packaging organizations (CPOs) segment is expected to register the highest CAGR in the pharmaceutical secondary packaging market during 2025-2034. The pharmaceutical business has changed over the past several decades, moving toward the practice of outsourcing not just production and packaging but also crucial services like product launch, e-pedigree services, medication marketing, and much more. They have collaborated with CPOs rather than investing in expensive packaging equipment, labor resources that are educated about packaging solutions, and buildings that have packaging activities.
North America dominated the pharmaceutical secondary packaging market in 2024. The aging population, rising healthcare costs, and the increasing value of U.S. pharmaceutical exports are some of the major reasons driving the growth of the pharmaceutical contract packaging sector in this region in the years to come. Additionally, the region's growth is expected to be greatly aided by the establishment of a number of important market participants, such as Recipharm, Ropack Inc., Reed-Lane Inc., Jones Healthcare Group, Almac Group Limited, and others.
The pharmaceutical sector in the United States is a world leader, exhibiting exceptional market domination and inventiveness. With total spending predicted to surpass $1 trillion by 2030, the pharmaceutical sector in the United States is at a turning point. Innovative therapies are the main driver of this expansion, especially in specialist domains like personalized medicine and cancer.
With a 2.2% global pharmaceutical market share, Canada ranks tenth in the world. Companies do research and development (R&D) to develop new or improved patented treatments, and some produce bio-equivalent forms of innovative drugs after patents expire. Two emerging fields of biopharmaceuticals are gene and cell therapies and nanomedicines. In Canada, brand-name products account for 80.5% of sales by value and 25.7% of prescriptions by quantity. Generics account for 19.5% of global sales by value and 74.3% of market share by prescriptions, per IQVIA Pharmafocus 2027.
Asia Pacific is estimated to host the fastest-growing pharmaceutical secondary packaging market during the forecast period. Pharmaceutical packaging is expanding quickly in the Asia Pacific area due to rising medical output and exports, as well as increased government backing for local manufacture. Adoption of smart packaging, sustainability, and traceability has become a key area of focus to support regional prosperity.
China's economy and market have taken center stage in the Asia-Pacific area as the world's second-biggest economy and fastest-growing nation. China's pharmaceutical sector contributes significantly to the country's economy due to its ongoing high demand, necessity, and capacity for innovation. The sector is still expanding at a rapid pace. The momentum assisting in the growth of China's pharmaceutical market is accelerating due to the country's growing focus on the pharmaceutical sector and the steady advancement of healthcare regulations.
During FY 2023–2024, the pharmaceutical market in India is projected to be valued at USD 50 billion, of which USD 23.5 billion would come from domestic consumption and USD 26.5 billion from exports. India's pharmaceutical industry is rated fifteenth in terms of production value and third in terms of volume worldwide. Because of its wide range of products, which includes biologics, biosimilars, vaccines, generic and bulk drugs, and over-the-counter pharmaceuticals, the Indian pharmaceutical industry is well-known worldwide.
Europe is expected to grow significantly in the pharmaceutical secondary packaging market during the forecast period. The established pharmaceutical industry and the rising need for environmentally friendly packaging options are driving the market in Europe. Recyclability and environmentally friendly products are prioritized in European nations. Furthermore, adherence to European Medicines Agency (EMA) requirements is crucial, resulting in advancements in patient-centered packaging, anti-counterfeiting strategies, and serialization.
In Q4 2024, the biopharmaceuticals and biologics industry in Germany maintained its growth trajectory, propelled by advancements and the growing market share of biosimilars. Major biologics' patent expirations created openings for biosimilars, which offered more affordable options. Technological developments in manufacturing and bioprocessing have simplified production procedures, lowering expenses and raising the caliber of output. With an emphasis on growing biosimilar portfolios and improving production capabilities, the industry is well-positioned for future expansion.
The UK continues to provide a substantial contribution to global medical sciences citations, coming in second only to China and the United States, with 11.5% in 2023. With 1.8% of publications rated as highly referenced—the greatest percentage of any comparison country and on par with France, Italy, and Germany—the UK led comparators in the production of high-caliber academic research in the medical sciences.

In January 2025, with our next-generation polymer syringe system, we are setting a new standard in the pharmaceutical industry by providing a solution that ensures drug stability, improves operational efficiency, and reduces valuable drug and packaging waste, said Andreas Reisse, CEO of SCHOTT Pharma. Our collaboration with Schreiner MediPharm and Körber Pharma has enabled us to create a solution that really meets the needs of patients, medical professionals, and our clients. (Source - Contract Pharma)
By Packaging Type
By Material Type
By Application
By End Use
By Region
November 2025
November 2025
November 2025
October 2025