January 2026

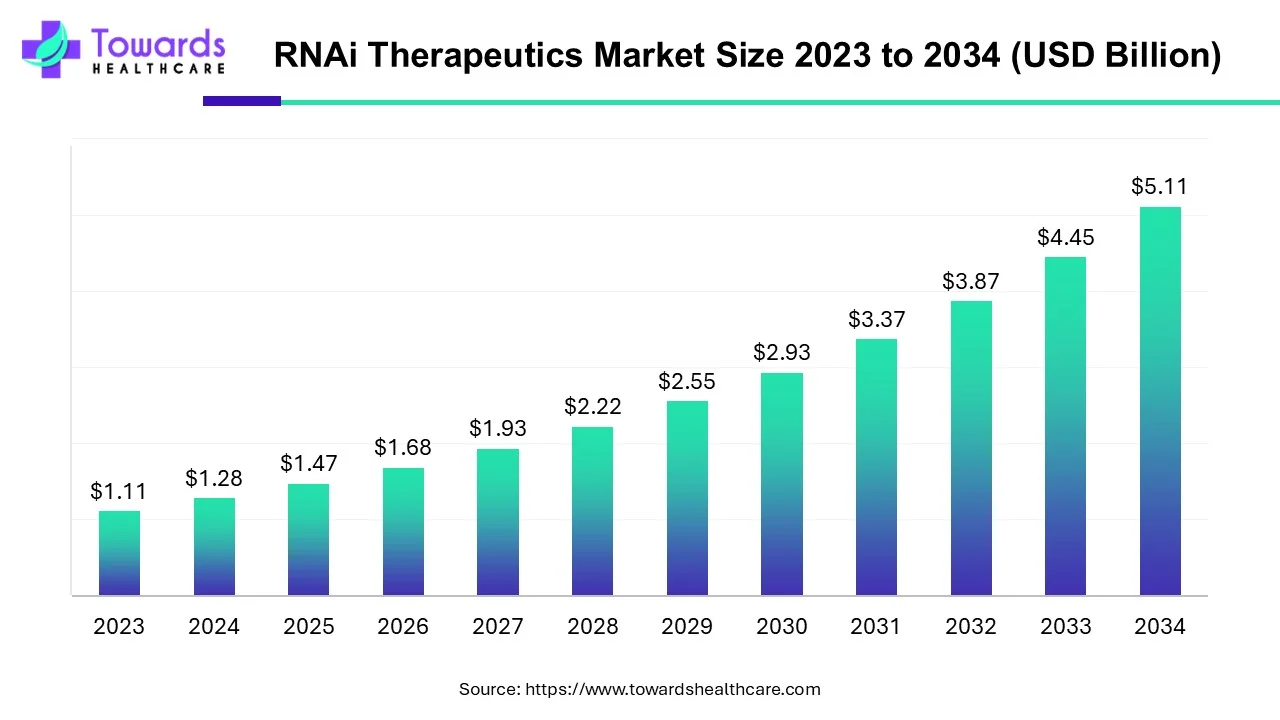
The RNAi therapeutics market is forecast to grow at a CAGR of 14.9%, from USD 1.47 billion in 2025 to USD 5.11 billion by 2034, over the forecast period from 2025 to 2034.
The American Cancer Society's report forecasts a substantial increase in both new cancer diagnoses and related deaths for 2022, with approximately 1.9 million new cases and 609,360 fatalities anticipated in the United States. RNAi therapeutics offer potential management for the escalating incidence of cancer.
In December 2020, a company expanded its collaboration efforts, focusing on developing and selling RNAi drugs. They utilized their advancements in delivering siRNA drugs directly to target areas and their existing skills to create RNAi drugs for further treating tumors and neurological conditions. This move could lead to more widespread use of RNAi in diagnosing diseases at the molecular level and make RNAi a more effective treatment option, potentially driving growth in the RNAi market. RNAi (RNA interference) therapeutics use small RNA molecules to silence or regulate specific genes within cells. These molecules, such as small interfering RNA (siRNA) or microRNA (miRNA), target messenger RNA (mRNA) to prevent the production of specific proteins, thereby influencing gene expression.
Recent News
RNAi therapeutics hold promise for treating various diseases, including genetic disorders, viral infections, and certain types of cancer. They can be delivered directly to target cells or tissues to modulate gene expression, offering a particular and potentially practical treatment approach.
One significant advantage of RNAi therapeutics is their ability to target disease-causing genes with high precision, potentially minimizing off-target effects and improving treatment outcomes. Additionally, RNAi technology enables the development of personalized medicine approaches tailored to individual patients' genetic profiles. RNAi therapeutics represent a cutting-edge approach in medicine with the potential to revolutionize the treatment of a wide range of diseases by targeting specific genes involved in their development and progression.
RNA interference (RNAi) therapeutics have garnered significant attention in clinical research due to their potential to revolutionize disease treatment. Numerous clinical trials are underway, focusing on various applications of RNAi technology across different medical conditions. These trials encompass genetic disorders, viral infections, neurodegenerative diseases, and cancer, among others. The precision and specificity of RNAi therapeutics offer promising avenues for targeted treatment approaches, addressing the underlying molecular mechanisms of diseases. As these clinical trials progress and demonstrate efficacy and safety, they pave the way for the market growth of RNAi therapeutics.
Moreover, advancements in delivery systems and RNAi molecule design contribute to the expanding landscape of RNAi-based treatments. With ongoing research and development efforts, RNAi therapeutics hold the potential to become a cornerstone in modern medicine, offering customized and practical solutions for a wide range of diseases.
The use of RNA interference (RNAi) therapeutics began to gain traction in clinical trials starting in 2008, a decade after the RNAi mechanism was discovered. Since then, the number of clinical trials involving RNAi-based drugs has surged. From 2008 to 2022, the annual number of published clinical trials using these therapeutics increased nearly tenfold, and the number of ongoing trials initiated between 2015 and 2021 grew about sevenfold.
However, there was a notable decline in ongoing trials in 2022, with a nearly 40% drop in studies using small interfering RNA (siRNA) drugs. Despite this, siRNAs have remained the most commonly used type of RNAi molecule in clinical trials. In both published and ongoing trials from 2008 to 2022, siRNAs were by far the most prevalent, comprising about 90% of trials in 2022. This trend has continued into 2023, with all published and ongoing trials focusing on siRNA-based drugs.
On the other hand, the use of short hairpin RNAs (shRNAs) has been less frequent but consistent in clinical trials over the years. shRNA-based treatments appear in a small but steady number of studies. Similarly, research involving microRNAs (miRNAs) and miR-shRNAs is limited, with few investigations published or ongoing.
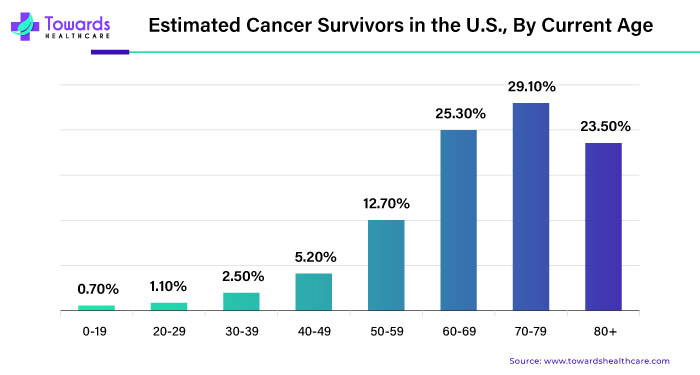
According to the American Cancer Society, around 1.92 million new cases of cancer and 609,360 deaths occurred in the United States alone in 2022. Globally, this adds up to a staggering 9.6 million lives lost. With the growing number of cancer diagnoses worldwide, there's a heightened need for groundbreaking treatments. RNA interference (RNAi) technology has emerged as a hopeful strategy in battling cancer.
Cancer is a disease where cells grow uncontrollably, forming tumors that can spread to other parts of the body. It's a serious health problem affecting millions of people globally.
Scientists have been exploring new ways to treat cancer more effectively. One such approach is using RNA interference (RNAi) technology. RNAi is a natural process in our bodies that helps regulate genes. By harnessing this process, scientists can develop treatments targeting the genes involved in cancer growth.
For instance,
These treatments, known as RNAi therapeutics, send small pieces of RNA to the cancer cells. Once inside the cells, these RNA pieces interfere with the genes responsible for cancer growth, essentially shutting them down.
One of the reasons why RNAi therapeutics are gaining popularity is because they offer a more targeted approach compared to traditional cancer treatments like chemotherapy. Conventional therapies often affect both cancerous and healthy cells, leading to various side effects. RNAi therapeutics, on the other hand, can specifically target cancer cells, minimizing damage to healthy tissues and reducing side effects.
As a result, the RNAi market is proliferating as more research is being done to develop and improve RNAi-based treatments for cancer. Pharmaceutical companies, research institutions, and startups invest heavily in this area, hoping to bring new and effective treatments to needy patients. The rising incidence of cancer has fueled the growth of the RNAi market, offering new hope for patients and doctors alike in the fight against this devastating disease.
The RNAi market undergoes growth across distinct developmental phases, from preclinical research to the approval of drugs for commercial use. In the preliminary preclinical research stage, scientists delve into the potential applications of RNA interference (RNAi) technology for therapeutic purposes. This phase involves rigorous laboratory experiments and animal studies aimed at unraveling the mechanisms of RNAi, crafting RNAi molecules, and assessing their effectiveness and safety profiles. As advancements in RNAi technology and preclinical research yield promising outcomes, there's a surge in interest and investment in RNAi-based therapeutics, propelling market expansion.
Transitioning from preclinical research, RNAi therapeutics progress into clinical trials to evaluate their safety, efficacy, and tolerability in human subjects. These trials unfold across multiple phases, commencing with Phase I, primarily focused on safety assessments, followed by Phase II, which delves into efficacy evaluations, and culminating in Phase III, where large-scale efficacy and safety studies are conducted. Positive results from these clinical trials demonstrate the potential of RNAi-based drugs to combat various diseases, further fueling market growth. Additionally, as RNAi therapeutics advance through clinical development and exhibit promising outcomes, they attract augmented investment and partnership opportunities from pharmaceutical companies and investors.
| Sr. No. | Target Gene | Drug Name | Route of Administration | Indication | Manufacturing Company | Reference |
| 1. | ALAS1 | ALN-AS1 (Givosiran) | Subcutaneous | Acute Hepatic Porphyrias | Alnylam Pharmaceutical | NCT03338816, |
| 2. | AT | Fitusiran ALN-AT3SC (Fitusiran) |
Subcutaneous | Hemophilia A or B | Genzyme, a Sanofi Company | NCT03417102/03417245, Completed |
| 3. | TP53 | QPI-1002 (Teprasiran) |
Intravenous | Prevention of acute kidney injury after cardiac surgery | Quark Pharmaceuticals | NCT03510897, Terminated |
| 4. | TRPV1 | SYL1001 (Tivanisiran) |
Periocular | Sjogren’s Syndrome, Dry eye (Eye) | Sylentis | NCT04819269, Recruiting |
| 5. | TRPV1 | SYL1001 (Tivanisiran) |
Periocular | Moderate to severe eye disease | Sylentis | NCT03108664, Completed |
Upon successful completion of clinical trials and regulatory approval, RNAi therapeutics undergo a pivotal transformation from experimental treatments to approved drugs. These drugs are then commercialized and accessible to patients to treat specific diseases. Approved RNAi drugs are pivotal in driving market growth by furnishing novel treatment options for individuals grappling with unmet medical needs. Furthermore, as the tally of approved RNAi drugs escalates, the market broadens, ushering in opportunities for further research, development, and innovation in RNAi-based therapeutics. With continued progress in research and development efforts, the RNAi market is poised for further expansion, promising innovative solutions for addressing a diverse array of diseases.
RNA interference (RNAi) is a natural biological process that regulates gene expression by suppressing the translation of specific messenger RNA (mRNA) molecules. The discovery of RNAi has led to the development of RNA-based therapeutics, particularly small interfering RNA (siRNA) and microRNA (miRNA), as potential treatments for various diseases, including cancer, viral infections, genetic disorders, and neurodegenerative diseases.
The clinical translation of RNAi therapeutics has encountered several challenges related to delivery. These challenges primarily stem from the inherent properties of RNA molecules and the physiological barriers they encounter in vivo. Additionally, RNA molecules are susceptible to degradation by nucleases in bodily fluids such as blood and tissue extracellular spaces, limiting their stability and bioavailability at the target site. Furthermore, RNA molecules may interact with unintended mRNA targets, leading to off-target effects and potential toxicity due to sequence similarity or nonspecific interactions with cellular components.
Introducing RNA molecules from outside the body can activate the immune system, mainly through pattern recognition receptors like retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and Toll-like receptors (TLRs). This immune response may lead to inflammation, the release of cytokines, and the removal of the therapeutic RNA, ultimately decreasing its effectiveness.
Moreover, efficient delivery of RNA molecules into target cells is critical for therapeutic efficacy, but they face barriers in crossing cellular membranes composed of hydrophobic lipid bilayers. Once inside the cell, RNA molecules must navigate through intracellular compartments to reach their site of action, such as the cytoplasm or nucleus.
To overcome these delivery challenges, researchers have developed various strategies and delivery vehicles, including lipid nanoparticles (LNPs), polymer-based nanoparticles, viral vectors, chemical modifications of RNA, and targeted delivery strategies. These approaches aim to protect RNA molecules from degradation, enhance their specificity, reduce off-target effects and immune activation, and improve their cellular uptake and intracellular trafficking. Addressing the challenges associated with RNAi therapeutics delivery is essential for realizing their full potential in clinical applications. Continued research efforts to develop innovative delivery platforms and optimize delivery strategies are crucial for advancing RNA-based therapies and improving patient outcomes.
North America, particularly the United States, is a significant hub for RNAi therapeutics development and commercialization. The region benefits from a robust biotechnology sector, vital research infrastructure, and supportive regulatory agencies like the Food and Drug Administration (FDA). Key players in the RNAi therapeutics market, including biopharmaceutical companies and academic institutions, are advancing RNAi-based treatments for diseases such as cancer, viral infections, and genetic disorders. The presence of well-established healthcare systems and high healthcare expenditure levels contribute to the adoption of innovative therapies, including RNAi therapeutics, in North America.
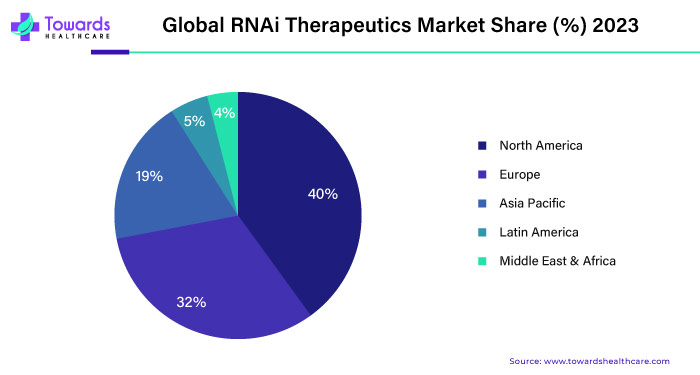
The Asia-Pacific region presents opportunities for growth in the RNAi therapeutics market, driven by factors such as increasing healthcare expenditure, expanding biopharmaceutical industry, and rising prevalence of chronic diseases. Countries like China, Japan, South Korea, and India are investing in biotechnology research and development, including RNAi-based technologies. Regulatory frameworks for RNAi therapeutics are evolving in the region, with authorities working to streamline approval processes and foster innovation.
Europe is expected to grow at a considerable rate in the RNAi therapeutics market in the upcoming period. Favorable regulatory policies and growing research and development activities drive the market. The European Medicines Agency (EMA) has approved 5 RNAi therapeutics. The European government also promotes the development of RNAi therapeutics through research funding. The rapidly expanding biopharmaceutical sector and the rising prevalence of several rare genetic disorders facilitate market growth.
Germany Market Trends
Approximately 4 million people are estimated to be living with a rare disease in Germany. The demand for RNAi therapeutics has increased since the launch of the first-ever RNAi therapeutic, Onpattro, in Germany by Alnylam Pharmaceuticals in 2018.
UK Market Trends
The UK government, in collaboration with Wellcome Trust, announced an investment of £600 million ($764 million) to build a centralized medical data repository to accelerate the development of new therapeutics. The policy also helps researchers get clinical trials set up within 150 days.
The competitive landscape of the RNAi therapeutics market is characterized by various companies and research institutions actively developing and commercializing RNA interference-based therapies. These entities compete in technology innovation, research and development pipelines, intellectual property, market access, and strategic partnerships. Emerging companies and startups are entering the RNAi therapeutics market with innovative technologies and therapeutic candidates. Collaborations between academia and industry are joint in the RNAi therapeutics space, facilitating technology transfer, access to funding, and expertise exchange. Companies with robust patent portfolios may have a competitive advantage in market exclusivity and the ability to enforce their intellectual property rights.

By Type
By Application
By Development Stage
By Geography
January 2026
November 2025
November 2025
December 2025