February 2026

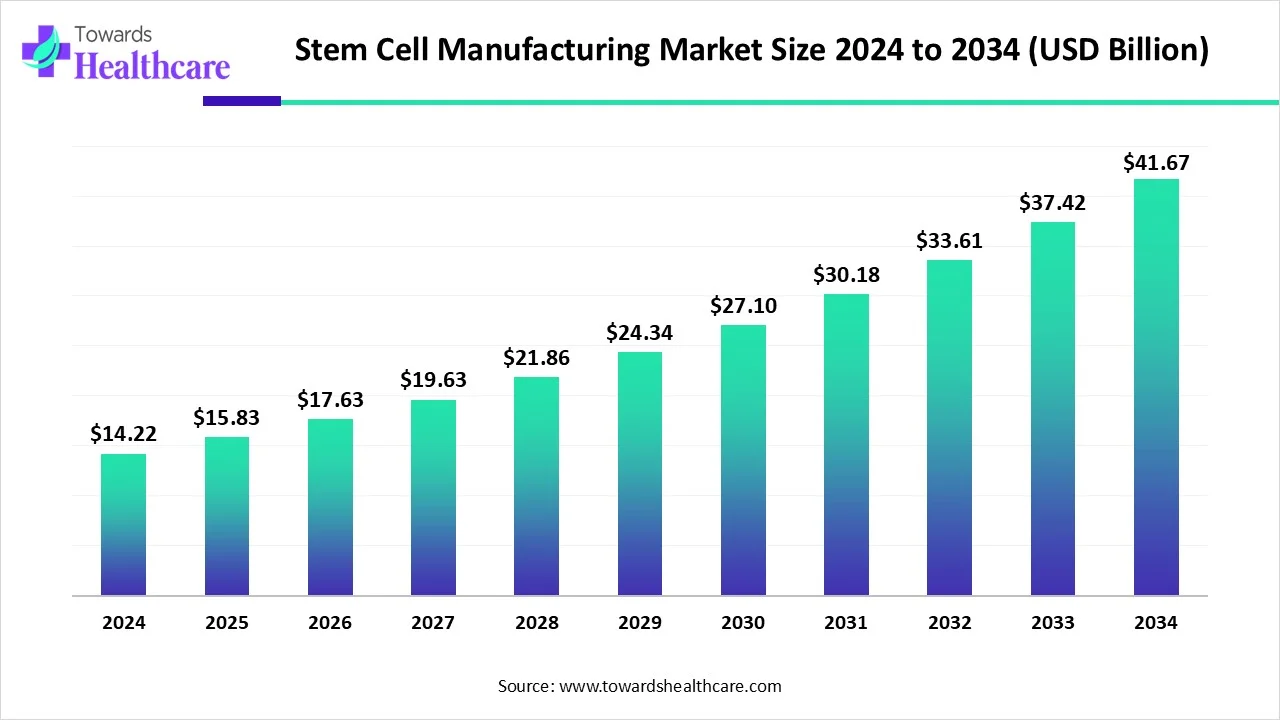
The global stem cell manufacturing market size is calculated at USD 14.22 in 2024, grew to USD 15.83 billion in 2025, and is projected to reach around USD 41.67 billion by 2034. The market is expanding at a CAGR of 11.35% between 2025 and 2034.
The manufacturing process is essential to the success of stem cell therapies because it guarantees the creation of safe and efficient stem cell products. The complicated process of isolating cells—either directly from the patient or from a donor—and then growing, differentiating, and preserving the differentiated cells is required to produce stem cell products. For the cells to retain their therapeutic qualities and to satisfy strict safety and quality requirements, this process needs to be closely monitored.
| Metric | Details |
| Market Size in 2024 | USD 14.22 Billion |
| Projected Market Size in 2034 | USD 41.67 Billion |
| CAGR (2025 - 2034) | 11.35% |
| Leading Region | North America |
| Market Segmentation | By Connectivity, By Indication, By Distribution Channel, By Region |
| Top Key Players | Novo Nordisk A/S, Ypsomed AG, Diabnext, Medtronic, Cambridge Consultants Ltd., Pendiq, Emperra GmbH, Jiangsu Deflu Medical Device Co. |
Improving the quality of stem cell manufacturing and distribution requires artificial intelligence (AI). Artificial Intelligence may be used to evaluate the safety, effectiveness, and viability of stem cells. In stem cell and regenerative medicine, artificial intelligence (AI) is also utilized to speed up simulation and model-building processes and find connections between cellular activity and its microenvironments. To minimize unforeseen repercussions, however, the use of AI in stem cell-based treatment requires careful consideration.
Rising Capital in Stem Cell Research
Stem cells are essential for the treatment of disease and for some types of research, such as genetic testing and personalized therapy. Globally, public and commercial stakeholders are spending more on the development of stem cell-based technologies as they grow more conscious of the therapeutic potential of stem cells and the dearth of medicines for orphan diseases. As rare genetic diseases become more widespread, the market for stem cell treatment is anticipated to grow. Every year, millions of people are impacted by rare diseases, including cystic fibrosis, phenylketonuria, thalassemia, and others.
Operational Cost
Stem cells are isolated, purified, and stored using expert procedures. High operating costs, in addition to the production of stem cells. As a result, stem cell products are usually more expensive than biologics and pharmaceutical medications.
Advancements in Regenerative Medicine
The need for stem cell manufacturing is mostly driven by the growing interest in regenerative medicine. The potential uses of stem cells are expanding thanks to new techniques and technology; for example, they can be used to manage chronic illnesses or create customized therapies. This increases the need for stem cell products and services in both clinical and scientific contexts and encourages further financing for stem cell research and development.
By product, the consumables segment dominated the stem cell manufacturing market in 2024. The need for these vital resources grows along with the amount of study being done in this area. In addition, the increasing number of regenerative medicine applications and stem cell clinical trials drives consumables consumption. The safety and effectiveness of stem cell-based therapies depend on a steady and dependable supply of high-quality consumables for clinical research and therapy development. Accordingly, technical developments in consumables manufacturing are propelling expansion.
By product, the instruments segment is estimated to grow at the fastest CAGR in the stem cell manufacturing market during the forecast period. A high demand for contemporary instruments has resulted from funding for research and development initiatives in stem cell biology and regenerative medicine. Due to stringent legal limitations on stem cell production, sophisticated tools are required to guarantee adherence to safety and quality requirements. High-quality, dependable equipment is necessary since instruments used in regulated environments must adhere to particular requirements.
By application, the clinical applications segment led the stem cell manufacturing market in 2024. The number of illnesses and patients receiving stem cell treatment has led to a considerable growth in the clinical uses of stem cells. Allogenic and autologous stem cells, adult stem cells, induced pluripotent stem cells (iPSCs), and both in vitro expanded and non-expanded stem cells were used in therapeutic settings. According to clinicaltrials.gov, stem cells are being used in over 5000 clinical studies to treat over 50 different illnesses.
By application, the research applications segment is expected to grow significantly in the stem cell manufacturing market during 2025-2034. Non-specialized cells called stem cells have the capacity to develop into a wide variety of cell types. Since their discovery, they have played a significant role in research because of their enormous potential to cure a wide range of incurable diseases with different linked medications or procedures. Stem cells are being utilized to investigate the influence of the environment on the formation of cells and tissues, as well as disorders including congenital heart disease and neurodevelopmental abnormalities. Each year, science and technology related to stem cells advance quickly.
By end-user, the pharmaceutical & biotechnology companies segment dominated the stem cell manufacturing market in 2024. Companies in the biotechnology and pharmaceutical industries have recently been more interested in stem cell biology. Since most large pharmaceutical firms employ adult or embryonic stem cells (ESCs) for internal drug development programs, the utilization of stem cells as research tools has increased. These internal initiatives are frequently strengthened by the knowledge gained from outside collaborations with academic institutions or biotech businesses.
By end-user, the academic institutes, research laboratories & contract research organizations segment is anticipated to grow at a significant rate in the stem cell manufacturing market during the forecast period. Government and corporate sector financing for stem cell research has increased. Governments, commercial establishments, and nonprofit groups recognize the revolutionary potential of stem cell research and offer funding to spur development. Other motivators include growing public knowledge of the likelihood of stem cell-based therapies and cooperative initiatives between academic institutions and industrial participants.
North America dominated the stem cell manufacturing market in 2024. Its high presence of top academic universities, significant biotechnology investments, and sophisticated healthcare infrastructure are the reasons for this. Innovation and development in this area are bolstered by increased financing from the public and commercial sectors. North America's favorable regulatory frameworks promote development and innovation by offering a favorable setting for clinical trials and stem cell research. The demand for stem cell therapies is further driven by a growing emphasis on personalized medicine, academic institutions and industry players working together, and public awareness of the likelihood of stem cell-based treatments. These factors have solidified North America's dominant position in the global stem cell manufacturing market.
In the United States, 4,099 medicines are in various stages of development, from preclinical to pre-registration. Currently in development are 2,042 gene therapies, which make up 49% of gene, cell, and RNA treatments. These include genetically modified cell therapies like CAR-T cell therapies. 22% of gene, cell, and RNA treatments are non-genetically modified cell therapies, of which 923 are now under development.
The foundation of Canada's economic prosperity and advancement is science and research. Researchers in Canada strive to increase our knowledge of the world and come up with fresh solutions to some of the most pressing problems of our day. The Canadian government takes pride in its investments in scientific and research institutions that are essential to the country's research environment. A higher standard of living for all Canadians will be ensured by the government's continued support of science and research, particularly via the Strategic Science Fund, which is solidifying Canada's standing as a global leader in innovation.
Asia Pacific is estimated to host the fastest-growing stem cell manufacturing market during the forecast period because of growing investments in biotechnology, the development of stem cell research projects, and the growing need for cutting-edge medical therapies. Significant developments in stem cell-based therapies are anticipated in nations like China, Japan, and South Korea, particularly as the healthcare systems in these areas expand and concentrate on more individualized and regenerative treatments. The industry is anticipated to gain from the region's growing emphasis on biopharmaceuticals, better healthcare facilities, and government funding for R&D.
China has stepped up its efforts to study and apply stem cells over the last five years. China now offers the most lenient and conducive conditions for the study of human embryonic stem cells. China is now the nation that is pursuing the field with the most vigor. Government subsidies are used in China to encourage research on adult stem cells and ESCs. The ambitious ambitions of the Chinese Ministry of Science and Technology to propel the nation to the forefront of research are well-suited to stem cell research. Through a variety of channels, including cities, provincial governments, and two unique national research projects (863 and 973 plans), China has invested heavily in this field.
Many agencies and institutions within the Indian government have conducted stem cell research. A cutting-edge research facility in Bangalore, India, the Institute for Stem Cell Biology and Regenerative Medicine (inStem) is devoted to the study of stem cells and regenerative biology. The focus of inStem, an independent institute supported by the Indian government's Department of Biotechnology, is cooperative stem cell biology research. The scope of inStem's research is broad and includes anything from clinical investigations into the effects of stem cells on stroke and injury rehabilitation to inquiries into the basic processes governing differentiation and renewal.
Europe is expected to grow significantly in the stem cell manufacturing market during the forecast period, distinguished by sophisticated healthcare infrastructure and robust research capabilities. The area gains from substantial investments in biotechnology research as well as established regulatory frameworks. The core of the European market is made up of nations like Germany, the United Kingdom, France, Italy, and Spain, each of which makes a distinct contribution to the industrial environment for stem cells in the area. The region's market is growing due to the presence of top research institutes and biotechnology businesses, as well as a growing emphasis on regenerative medicine.
From molecular level science to public health medicine, UKRI's Medical Research Council (MRC) funds the greatest scientific research to enhance human health. MRC is crucial in providing financing for foundational studies that may not be directly related to a particular illness but will advance medical research in general. A sizable portfolio of researcher-led initiatives is produced by UKRI. This covers a broad range of topics, such as physiological, biochemical, and mechanistic elements that are relevant to several illnesses, disorders, and other circumstances.
In May 2024, in addition to providing affordable solutions to pharmaceutical, biotechnology, and therapeutic companies, REPROCELL will manufacture commercially available GMP-grade hiPSC and hMSC for internal use. The CEO of the global REPROCELL firm, Dr. Chikafumi Yokoyama, claims that because of its GMP Manufacturing Facility, REPROCELL is a pioneer in providing hiPSC from donor to GMP quality hiPSC MCB utilizing footprint-free RNA reprogramming technology. This GMP facility provides a unique resource for all cell therapy companies in Maryland and the rest of the United States, said Rama Modali, CEO of REPROCELL USA.
By Product
By Application
By End-user
By Region
February 2026
February 2026
February 2026
February 2026