January 2026

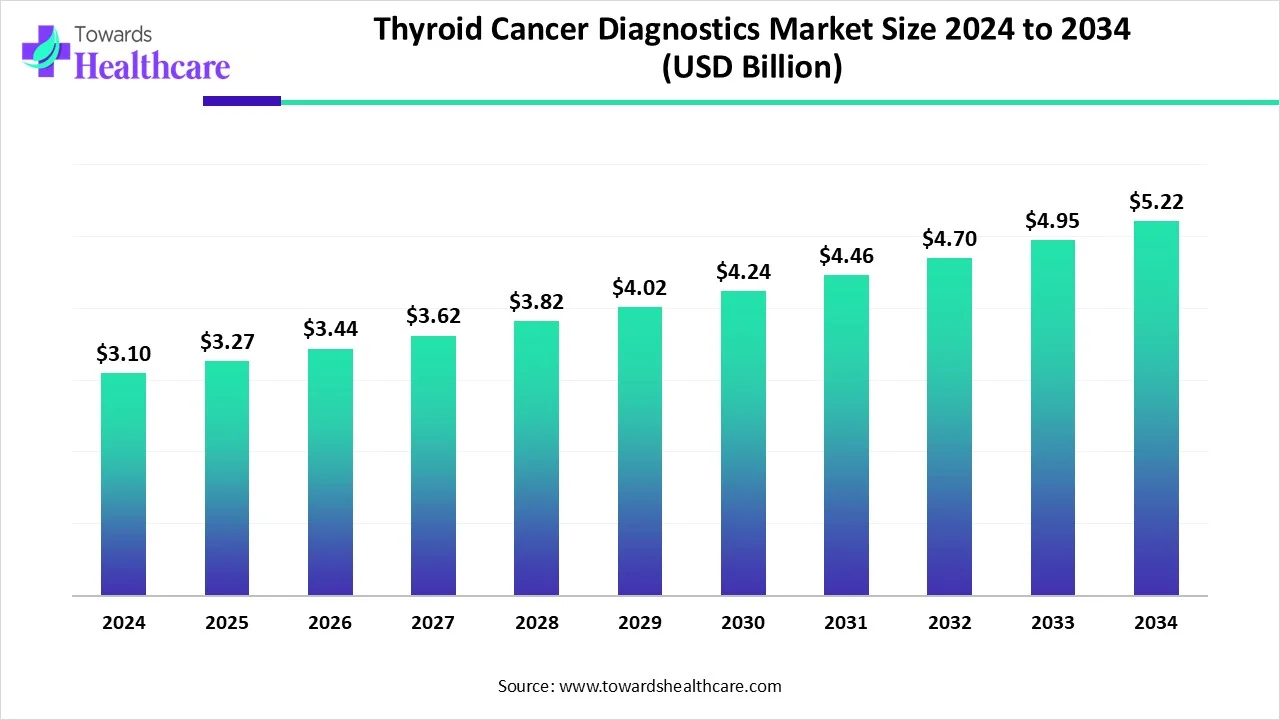
The global thyroid cancer diagnostics market size is calculated at USD 3.1 in 2024, grew to USD 3.27 billion in 2025, and is projected to reach around USD 5.22 billion by 2034. The market is expanding at a CAGR of 5.34% between 2025 and 2034.
The thyroid cancer diagnostics market is rapidly growing due to increasing thyroid disorder cases, growing awareness, and expanding healthcare infrastructure. Growing government support, technological developments in biopsy and imaging techniques. In North America growing demand for early detection methods, which drives the growth of the market. Growing medical tourism in the Asia Pacific contributes to the growth of the market.
| Metric | Details |
| Market Size in 2025 | USD 3.27 Billion |
| Projected Market Size in 2034 | USD 5.22 billion |
| CAGR (2025 - 2034) | 5.34% |
| Leading Region | North America share by 44% |
| Market Segmentation | By Cancer Type, By Technique, By End-use, By Region |
| Top Key Players | Abbott, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific, Inc., Siemens Healthcare GmbH, Bio-Rad Laboratories, Inc, GE HealthCare, Hologic, Inc., Koninklijke Philips N.V., Toshiba Corporation, Agilent Technologies, Inc., Illumina, Inc. |
The thyroid cancer diagnostics market is growing rapidly due to the recent advances in diagnostics technology of thyroid cancer, providing accurate imaging, treatment planning, and early detection. Thyroid cancer is diagnosed by using various methods, including blood tests, physical exams, biopsies, and recent techniques such as CT, PET, and MRI scans. Early detection is significant to managing thyroid health and effective treatment. Thyroid cancer arises when irregular growth of cells starts in the gland. This situation might not show any symptoms at first, but with time, it worsens and causes signs and symptoms, like voice changes, swelling in the neck area, and challenges in swallowing.
Undertaking regular healthcare check-ups supports the diagnosis of the condition at an early stage. This is the fastest-growing cancer diagnosis worldwide, predominantly among women. Additionally, major thyroid cancers are highly treatable; precise diagnosis is significant to determine the exact treatment approach. Molecular testing is an advanced diagnostic test that mainly involves analyzing the genetic material (DNA, specific proteins, and RNA) from thyroid cells or tissue to detect mutations or other biomarkers linked to cancer.
AI Integration in thyroid cancer diagnostics is driving the growth of the market as the role of AI-driven technology, such as machine learning and deep learning, integrated in the computer-aided diagnosis devices is to assist physicians in the diagnostic process, improve lesion recognition and evaluation, while sometimes revealing surprising features. An AI-based model is intended to classify both the risk category and cancer stage of thyroid cancer, attaining impressive accuracy. AI has been broadly applied in the detection of malignancy in thyroid nodules by ultrasound images, molecular markers, and cytopathology images. AI-based technologies are revolutionising the management and diagnosis of thyroid cancer in recent years, as in other domains of clinical care and medical practice. The development of advanced scientific technology in this field is expected to further improve AI technology, which drives the growth of the thyroid cancer diagnostics market.
Increasing Incidence of Thyroid Cancer
The growing incidence of thyroid cancer is due to a combination of an apparent rise in more sensitive diagnostic procedures and a possible consequence of increased population exposure to radiation and to various still-unrecognized carcinogens. This type of cancer is the most common endocrine cancer, and its occurrence has incessantly increased in the last decades globally. Major factors in the environment also cause growth in thyroid cancer and thyroid nodules, including the release of radiation from nuclear reactor accidents, which causes growth of the thyroid cancer diagnostics market.
Challenges of High Cost of Diagnostic Testing
The issue of test costs is especially pertinent for thyroid cancer (TC), given the rising incidence of thyroid nodules (TN), which is now recognized as a global pandemic. Individuals in upper-middle, lower-middle, and particularly low-income countries face significant personal financial strain, as the expenses for these advanced molecular tests are covered by general taxation and out-of-pocket payments.
Increasing Advancement in Analytical Techniques
Recent advances in the diagnosis and treatment of differentiated thyroid cancers have emerged. These contain molecular diagnostics and more advanced strategies for challenges stratification. Outstanding advances have occurred in numerous major biological areas of thyroid cancer, including the molecular alterations for the loss of radioiodine avidity of thyroid cancer, the pathogenic role of the MAP kinase and PI3K/Akt pathways and their associated genetic alterations, and the aberrant methylation of functionally important genes in thyroid pathogenesis and tumorigenesis. Recognition of these advanced features is significant to the management of patients with thyroid cancer. Advancements in treatments are being designed based on improved understanding of the thyroid cancer diagnostics process, which creates an opportunity for the thyroid cancer diagnostics market.
By cancer type, the papillary carcinoma segment dominated the thyroid cancer diagnostics market in 2024, as papillary thyroid carcinoma is an epithelial malignancy screening evidence of follicular cell differentiation and distinctive nuclear structures. It is the utmost frequent thyroid neoplasm and carries the overall prognosis. The tumor generally appears as an asymmetrical thyroid solid mass, but it may have cystic features in rare cases. Papillary carcinoma of the thyroid is the common cancer of the thyroid gland. The diagnosis and treatment of papillary carcinoma are made by fine needle aspiration (FNA) biopsy.
The follicular carcinoma segment is expected to grow at a significant CAGR over the forecast period, as this type of carcinoma is a highly treatable and curable cancer of the thyroid gland. Treatment habitually includes surgery, such as thyroidectomy. The diagnosis of follicular thyroid cancer in the thyroid gland can only be made by complete elimination of the thyroid mass and then microscopic inspection of the mass in the thyroid gland. The microscopic identification of these cells of the thyroid, called follicular cells, invading lymphatic vessels, blood vessels, or the capsule of the thyroid mass, is needed for the diagnosis of follicular thyroid cancer.
By technique, the imaging technique segment was dominant in the thyroid cancer diagnostics market in 2024, as the imaging techniques include magnetic resonance imaging and computerized tomography, both have a significant role in the evaluation of thyroid cancer. Also, Magnetic resonance imaging is superior to electronic tomography for the evaluation of retrotracheal, metastatic, or mediastinal involvement of large thyroid goiters or tumors. Careful choice of the diagnostic techniques will confirm a more precise diagnosis and reduce unnecessary patient expenses in the treatment of thyroid cancer. An imaging technique that uses a radioactive tracer.
Biopsy technique is expected to grow at a significant CAGR over the forecast period, as thyroid biopsy is a significant healthcare procedure for diagnosing different thyroid conditions, including thyroiditis, thyroid nodules, and thyroid carcinoma. A thyroid biopsy is a significant inspection to assess thyroid nodules and other thyroid disorders. It supports determining whether a nodule is benign or malignant, permitting physicians to plan the greatest suitable treatment.
By end-use, the hospital laboratories segment is dominant in the thyroid cancer diagnostics market in 2024, as hospital laboratories perform high-precision levels of thyroid cancer surgery, which benefits the patient with huge advantages such as reduced blood loss and pain, fewer treatment-related complications, reduced hospital stays, and quicker recovery. It supports mitigating or even eliminating symptoms that persist despite other treatments. Removing the thyroid reductions, the possibility of recurrence or relapse in thyroid-associated disorders.
The research institutes segment is expected to grow significantly in the market during the forecast period due to the many cancer institutes that have easy access to advanced diagnostic and therapeutic tools. Immunotherapy and targeted therapy are novel therapies shown to improve patient results, and cancer centers are equipped to provide them. Cancer institutes are at the forefront of implementing and developing these medicines, which have shown promise in treating and managing many forms of thyroid cancer.
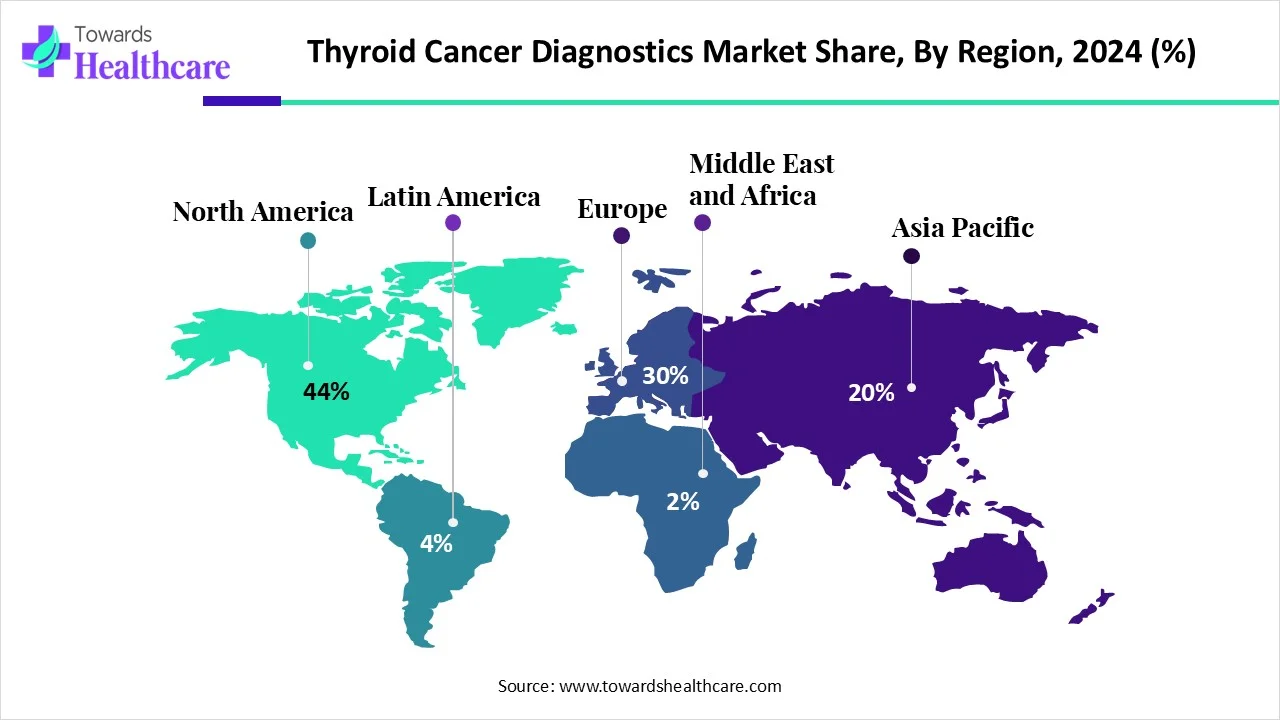
North America thyroid cancer diagnostics market dominated the global market with the largest revenue share by 44%, as the increasing cancer health awareness through the screening and education (CHANGE) initiative was designed to progress cancer care and lessen racial inequities and disparities. Increasing evidence‐based cancer advance technology through education and health literacy, with a prominence on prevention of modifiable risk factors, early detection, and screening, drives the growth of the thyroid cancer diagnostics market.
For Instance,
Increasing cancer rates are due to obesity, sedentary lifestyles, and highly processed foods, which are an epidemic in the U.S. The number of people existing in the U.S. with a history of cancer is increasing due to the aging and growth of the population, so developments in early detection and treatment, which improve rates of survival. In 2025, there will be about 44,020 fresh diagnoses of thyroid cancer, making it the ninth most common cancer in women in the U.S.
In Canada, it is the seventh most common cancer in women, with 6600 recent cases in 2024, which contributes to the growth of the market. Radiation is a major risk factor for thyroid cancer, and many physicians suggest that increased radiation exposure is driving the growing incidence in Canada. Thyroid cancer in Canada is associated with more severe diagnostic activities, driving the growth of the thyroid cancer diagnostics market.
The Asia Pacific region is projected to experience the fastest growth in the market during the forecast period due as the people in the Asia-Pacific region are adopting sedentary lifestyle habits that significantly increase the risk of thyroid cancer. The quick increase in cancer in the Asia-Pacific countries places an enormous burden on the Asia-Pacific society as a whole, which increases the demand for thyroid cancer diagnostics solutions. The APAC healthcare sector has strong potential for an increase in medical facilities and biotech companies that drive innovation. Growing disposable incomes are driving healthcare spending, which drives the growth of the market.
For Instance,
China has made dramatic achievements in the treatment and early diagnosis of cancer, as shown by its prolonged screening coverage and increased funding. The Chinese government has made extensive progress, and rich cancer screening information is being accumulated. Advances in technology have significantly improved diagnostic treatment and accuracy planning, driving the growth of the market.
Advanced technology is crucial in revolutionizing cancer care by facilitating early detection, tailored treatment strategies, minimally invasive techniques, accurate radiation therapy administration, effective data management and analysis, and remote patient monitoring. The swift adoption of fine-needle aspiration biopsy, ultrasound, MRI, and CT imaging in India is propelling the expansion of the market.
The European market is anticipated to experience considerable growth during the forecast period due to Europe's notably higher incidence and mortality rates from cancer. These factors drive the expansion of the thyroid cancer diagnostics sector. Significant advancements in imaging technologies, along with the application of artificial intelligence and supportive medical devices, enhance diagnosis capabilities, enabling earlier detection of patients' conditions.

In January 2025, Shahe Bagerdjian, President of INIS, stated, “We're glad to have agreed with Mr. Bono to finalize the Asset Purchase Agreement. It's good to now have full control of the intellectual property behind the Swirler and Tru-Fit, and we look forward to the Xenon System joining the rest of our RadVent medical device lineup in 2025.” (Source - Firstword Pharma)
By Cancer Type
By Technique
By End-use
By Region
January 2026
January 2026
December 2025
December 2025