January 2026

The global biopharmaceuticals CRO market is on an upward trajectory, poised to generate substantial revenue growth, potentially climbing into the hundreds of millions over the forecast years from 2025 to 2034. This surge is attributed to evolving consumer preferences and technological advancements reshaping the industry.
The biopharmaceuticals CRO market is experiencing increasing collaborations between companies due to growing drug developments as well as clinical trials. At the same time, the growing use of AI in these CROs is enhancing the workflow, minimizing errors, as well as maintaining privacy, which is attracting various companies, contributing to the market growth.
Moreover, well-developed or advancing industries, as well as growing research, are increasing the use of CRO services, achieving new agreements in different regions. Thus, all these factors enhance the partnerships, promoting market growth.
A company that offers outsourced research services with contracts is known as a contract research organization. Services such as clinical trial management, biopharmaceutical development, clinical development, biological assay development, real-world evidence, pharmacovigilance, and outcomes research are provided by these CROs. At the same time, they support the pharmaceutical and biotechnology industries. Thus, these biopharmaceutical CROs help in reducing the cost, drug development, as well as the entry of the drug into the market. Furthermore, they also support governmental organizations, universities, and research institutions.
The use as well as the adoption of AI in biopharmaceutical CROs, site management organizations (SMOs), as well as by clinical trial sponsors is increasing. This, in turn, is due to the growing applications of AI in patient recruitment, study monitoring, patient engagement, protocol development, site identification, as well as in study data review. Furthermore, they can also be used in biosimulation or in virtual trials. At the same time, the rules for improving confidentiality, privacy, as well as security are highly regulated, which increases the use of AI in the biopharmaceutical CROs.
Rising Diseases
The increasing diseases, such as cancer, diabetes, rare diseases, infectious diseases, etc., are increasing the demand for the development of new diagnostic and biopharmaceutical treatment approaches. Hence, this, in turn, contributes to the growing development of industries. This leads to the growing demand for biopharmaceutical CRO services. These services offer support in the trial management costs associated, as well as regulatory support. Furthermore, suitable infrastructure for various biologics is also provided by these services. Thus, all these factors drive the biopharmaceuticals CRO market growth.
Regulatory Constraints
Various strict rules and regulations imposed by the regulatory bodies for CROs affect the clinical trials time as well as cost. Furthermore, as the different regulations increase, the complexities also increase, which may result in errors. This, in turn, increases the demand for expertise to minimize errors as well as failure to comply with the regulations. Thus, regulatory constraints affect the market growth.
Growing Clinical Trials
The increasing incidence of diseases has led to the development of new biopharmaceutical products. At the same time, new therapies are also being developed. Thus, these developments contribute to the growing clinical trials. This, in turn, increases the demand for biopharmaceutical CRO services. Hence, with the help of these services, the complexities in the trials are minimized. Moreover, it also helps in site selection, regulatory data submission, expertise, as well as helps in reducing the trial costs. Thus, these services provided promote the biopharmaceuticals CRO market growth.
For instance,
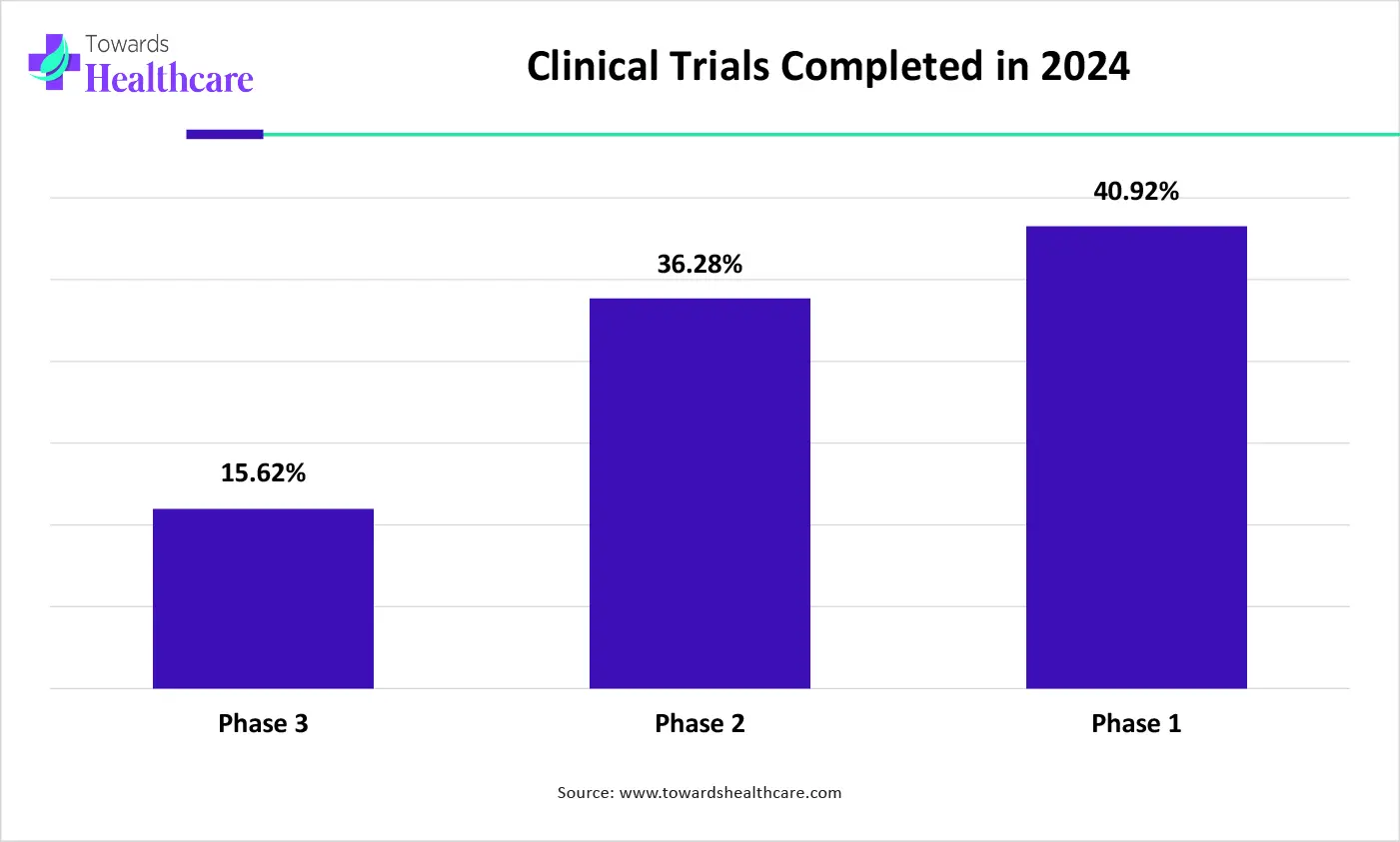
The graph represents the percentage of completed clinical trials in different phases in the year of 2024. It indicates that there is a continuous rise in the clinical trials. Hence, it increases the demand for biopharmaceutical CROs for the effective management of these trials. Thus, this in turn will ultimately promote the market growth.
North America dominated the biopharmaceuticals CRO market in 2024. North America consists of advanced biopharmaceutical industries, which increase the number of research and trials, enhancing the CRO contracts. Thus, this promotes the market growth.
The biopharmaceutical industries in the U.S. consist of advanced technologies, as well as skilled personnel, which accelerate the production of new drugs. Thus, this results in new biopharmaceutical CRO collaborations.
Due to growing advancements in the development of new diagnostic and treatment approaches in biopharmaceutical companies, the number of CRO services is also being attracted, which helps in their clinical trials.
Asia Pacific is expected to host the fastest-growing biopharmaceuticals CRO market during the forecast period. The industries in Asia Pacific are advancing due to growing development in diagnostics, treatments, as well as in infrastructure. At the same time, the biopharmaceutical CROs are also increasing, which are being supported by the government. Thus, this enhances the market growth.
The adoption of various technologies for biopharmaceutical product innovation in China is growing. This, in turn, increases the clinical trials, increasing the demand for biopharmaceutical CROs.
India is experiencing a rise in the development of industries, which increases the use of advanced technologies as well as the drug development process. This, in turn, attracts the CROs for the management of clinical trials. These are further supported by the government.
Europe is expected to grow significantly in the biopharmaceuticals CRO market during the forecast period. The growing research in Europe is increasing the number of drug development processes, contributing to rising clinical trials. Thus, to support these trials, the demand for biopharmaceutical CRO is increasing. This amplifies the market growth.
The demand for biopharmaceutical CRO services in Germany is growing due to increasing research and clinical trials. The growing incidence of diseases is causing a rise in the development of various biopharmaceutical products, enhancing their use.
The growing research in industries of the UK, is increasing the contract with biopharmaceutical CROs for various services. This, in turn, helps in increasing the successful clinical trial rates, which are supported by the government as well.
The Middle East & Africa are expected to grow at a notable CAGR in the biopharmaceuticals CRO market in the foreseeable future. The market is experiencing significant growth with the growing need for biologics and the increasing number of biopharma startups. The rapidly expanding biopharma sector bolsters the development of innovative personalized medicines. This is supported by several funding programs by government bodies. The increasing collaborations between key players and CROs contribute to market growth.
The UAE is home to 71 biopharma outsourcing startups. Dubai is a primary hub for life science companies, accounting for over 350 pharma firms. In December 2024, Emirates Biotech announced the construction of a new PLA production plant in Abu Dhabi. After the first phase, the plant boasts an annual capacity of 80,000 tonnes, and another annual capacity of 80,000 tonnes after the second phase.
Companies like ArabMed CRO, ClinServ International, and PDC CRO are major biopharmaceutical CROs in Saudi Arabia. There are around 19 biopharma outsourcing startups in Saudi Arabia. The Saudi Arabian regulatory agency has approved a total of 38 biosimilars for various purposes, owing to the increasing demand for cost-effective therapies.
By Region
January 2026
January 2026
December 2025
November 2025