January 2026

The gene editing therapeutics market is rapidly advancing on a global scale, with expectations of accumulating hundreds of millions in revenue between 2025 and 2034. Market forecasts suggest robust development fueled by increased investments, innovation, and rising demand across various industries.
The demand for gene editing therapeutics is increasing for the treatment of rare and genetic diseases. This is increasing their development to offer curative therapies for the same. These developments are being accelerated with the growing investments and acquisitions. AI is also being used to enhance its accuracy and applications. The growing research and development, and manufacturing capabilities are increasing their production across various regions. The companies are collaborating, promoting clinical trials and funding rounds to support these developments. Thus, this promotes the market growth.
The gene-editing therapeutics market covers the discovery, development, manufacturing, and commercialization of therapies that alter DNA or its expression in cells using programmable nuclease or template-based technologies (CRISPR/Cas systems, base editors, prime editors, zinc-finger nucleases, TALENs, ARCUS, meganucleases, and related delivery platforms). Applications include ex vivo edited cell therapies (CAR-T, HSC edits), in vivo editing (AAV/LNP/viral and non-viral delivery), and emerging precision therapeutic modalities (base and prime editing for single-base corrections). Market drivers are expanding clinical-stage pipelines, successful proof-of-concept readouts in rare diseases and hemoglobinopathies, improving delivery systems, growing manufacturing/CDMO capacity, and rising payer/health-system interest in curative or durable medicines.
Growing demand for curative therapies: Due to the growing genetic diseases, there is a rise in the demand for curative therapies. Hence, new gene editing therapeutics are being developed. Moreover, to accelerate their development, the number of investments as well as acquisitions is increasing.
For instance,
The therapeutic innovation and biomedical research are being transformed by integrating gene-editing technology with AI. The detection of genetic targets and the development of personalized medicine can be enhanced with the use of AI. The safety of gene-editing approaches can be improved by using machine learning algorithms in CRISPR research, as it helps in predicting off-target and on-target effects. The efficiency and specificity of genome editing can be enhanced by AI by promoting the selection and design of guide RNAs (gRNAs). Moreover, AI-powered models for gene-editing precision, discovery of novel CRISPR-associated enzymes, and mitigation of off-target effects are also being developed.
Growing CRISPR-Based ex Vivo Programs
There is a rise in the development of gene editing therapeutics due to growing CRISPR-based ex vivo programs. It helps in enhancing the quality control and precision of the process. This improves the safety and therapeutic consistency of the products. It also reduces the chances of risk as it involves the editing of the cell outside the body. It also helps in the development of personalized therapies. Moreover, the growing use of CAR-T therapies is increasing the demand for gene editing technologies. Thus, this drives the gene-editing therapeutics market growth.
Intricate Manufacturing
For the development of gene-editing therapeutics, sophisticated instruments, reagents, as well as a sterile environment are required. Most of the therapeutics developed are personalized, which results in small batch production. Additionally, the presence of multiple steps for their development increases the risk of batch failure or contamination. Thus, these factors make their development costly as well as complex.
Growing Applications
Due to growing diseases, the demand as well as the use of gene-editing therapeutics is increasing. They can provide targeted action at the DNA level. This helps in altering or removing the harmful gene or mutations. At the same time, diseases such as neurodegenerative diseases can be treated with these therapeutics. This, in turn, is increasing their use and development for targeting the root cause of the diseases. Moreover, their use for the treatment of rare diseases, cancer, neurological disorders, etc., is increasing. Thus, this promotes the gene-editing therapeutics market growth.
For instance,
By editing modality/platform type, the CRISPR/Cas segment held the largest share of approximately 48% in the market in 2024 due to its easy use. It offered high precision for targeting specific DNA sequences. It also provided the broadest clinical pipeline across ex vivo and in vivo programs. Thus, this contributed to the market growth.
By editing modality/platform type, the base editing/prime editing segment is expected to show the fastest growth rate during the forecast period. It offers enhanced safety with lower toxicity. It also provides single-base correction approaches for precision repair. This is increasing their use in the treatment of genetic diseases or sickle cell anemia.
By therapy type, the ex-vivo edited cell therapies segment led the market with approximately a 53% share in 2024, as it provided a controlled environment. They offer improved safety validation and editing efficiency. Moreover, their quality checks also increased the chances of achieving the desired action. They also reduced the side effects and risk of immune reactions, which increased their use.
By therapy type, the in-vivo gene-editing therapeutics segment is expected to show the highest growth during the forecast period. It offers a less invasive approach. Their use for the treatment of monogenic disorders is increasing. Furthermore, LNP/AAV delivered editing for liver, eye, CNS, and muscle is also being developed.
By indication/therapeutic area type, the genetic & rare diseases segment held the dominating share of approximately 40% in the market in 2024, because it provides specific genes as the targets. This, in turn, increased the use of gene editing therapies for developing potential treatment options. They were also used for the treatment of hemoglobinopathies, retinal dystrophies, and metabolic disorders.
By indication/therapeutic area type, the cardiovascular segment is expected to show the fastest growth rate during the forecast period. The growing cases of cardiovascular diseases are increasing the use of gene editing therapeutics. They can be used to minimize the risk of these diseases by lowering the cholesterol level. Moreover, in vivo editing for lipid targets is also being used.
By delivery modality type, the ex-vivo segment led the market with approximately a 55 % share in 2024, as it proved controlled editing. This, in turn, increased the editing efficiency and accuracy. They were used for immune-related or blood-related diseases. Thus, this promoted the market growth.
By delivery modality type, the lipid nanoparticles (LNP) & non-viral systems segment is expected to show the highest growth during the forecast period. They provide safe action with low side effects. They can offer tissue-specific action. Moreover, due to their easy manufacturing, their development is increasing for treating brain or liver-related diseases.
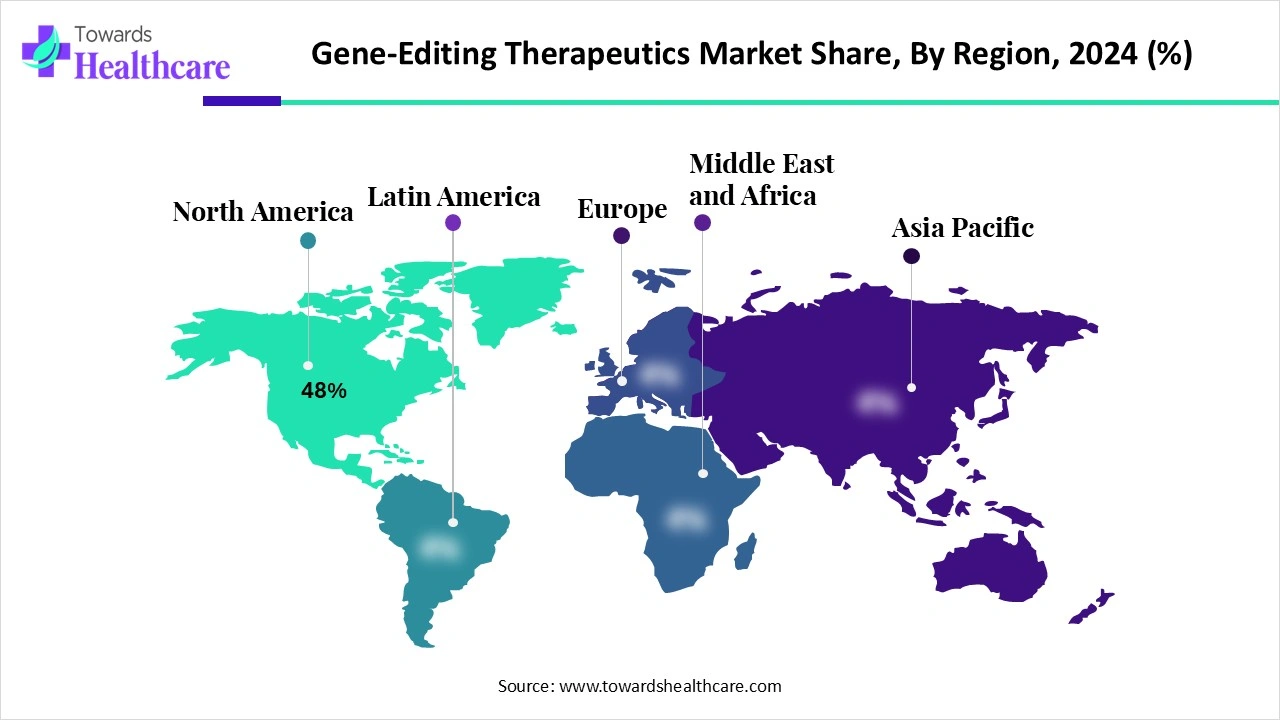
North America dominated the gene-editing therapeutics market share 48% in 2024. North America consists of well-developed industries, with an increased focus on the research and development of gene editing therapeutics. These developments were supported by a large number of sponsors promoting the use of gene editing tools. Thus, this contributed to the market growth.
The industries as well as institutes in the U.S. are increasing the development of gene editing therapeutics. At the same time, new tools such as base editing and prime editing are also being used. Moreover, the presence of a large number of clinical trial sites is also increasing their use. These developments are also being supported by various investments and funding.
There is a rise in research and development in Canadian institutes focusing on gene editing therapeutics and regenerative medicines. These R&D are further supported by the government funding or initiatives. Moreover, the growing startups are also contributing to these developments. Additionally, growing collaborations are also accelerating the gene editing therapeutics development.
Asia Pacific is expected to host the fastest-growing gene-editing therapeutics market during the forecast period. There is a rise in research and development in the Asia Pacific, which in turn increases the development of gene editing therapeutics. These developments are leading to a growth in their clinical trials. Moreover, the demand for cell therapy is increasing the demand for gene editing technologies, enhancing the manufacturing capabilities. These developments are also being supported by government investments. Thus, this enhances the market growth.
The R&D of gene editing therapeutics is focusing on developing safe and effective therapies for genetic and other diseases, along with growing advances in technologies like CRISPR, supported by investments.
Key Players: CRISPR Therapeutics, Intellia Therapeutics, Beam Therapeutics, Caribou Biosciences, Editas Medicine
The formulation and final dosage preparation focus on encapsulating gene-editing components into a delivery vehicle for in vivo or ex vivo administration.
Key Players: Intellia Therapeutics, CRISPR Therapeutics, Moderna Therapeutics, Beam Therapeutics, Prime Medicine
The personalized programs that address the emotional, logistical, and financial challenges of the treatment are involved in the patient support and services of gene-editing therapeutics.
Key Players: Vertex Pharmaceuticals, Bluebird Bio, CRISPR Therapeutics, Novartis Gene Therapies

In August 2025, after announcing the closing of the seed funding round of Axelyf, its CEO and co-founder, Örn Almarsson, stated that, to tackle the complex diseases, they are developing real solutions and not chasing science fiction. The RNA medicine next chapter will be unlocked with the use of their AXL technology, which offers exceptional performance, the team expertise, and AI-informed capabilities. Thus, this helps in the development of transformative products for them as well as their partners.
By Editing Modality/Platform
By Therapy Type
By Indication / Therapeutic Area
By Delivery Modality
By Region
January 2026
November 2025
November 2025
December 2025