February 2026

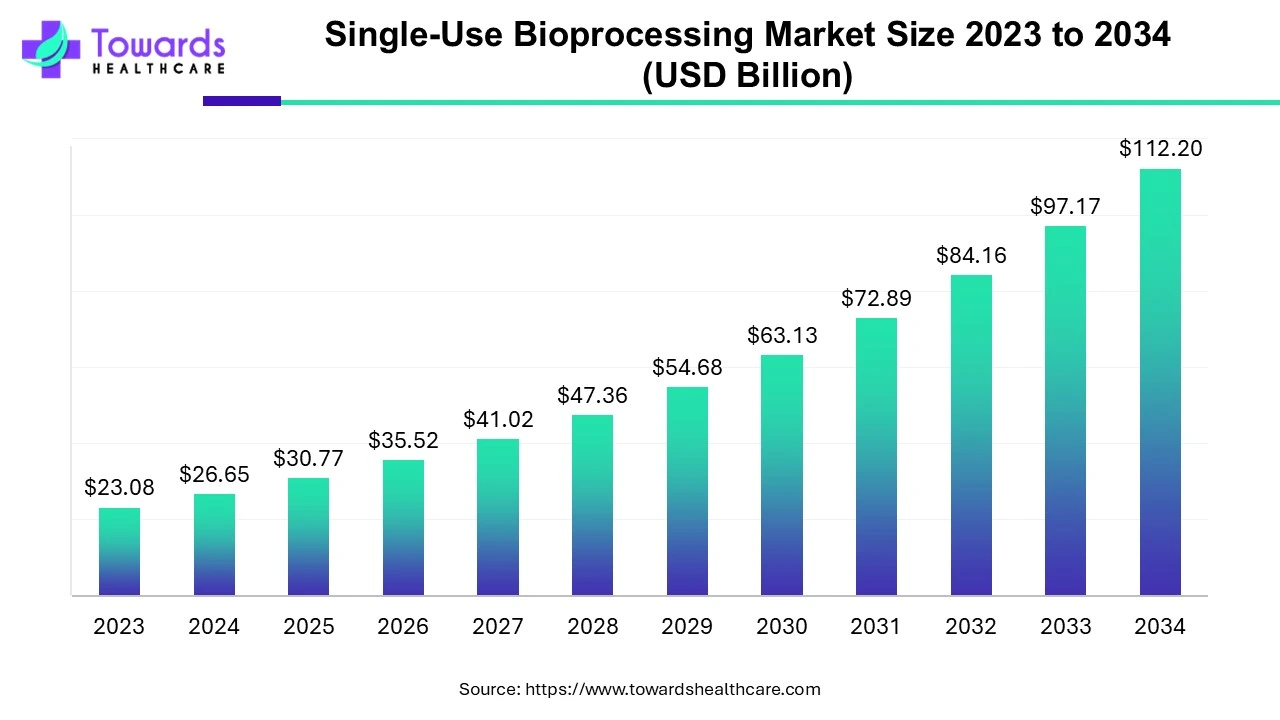
The global single-use bioprocessing market is expected to increase from USD 30.77 billion in 2025 to USD 112.2 billion by 2034, growing at a CAGR of 15.46% throughout the forecast period from 2025 to 2034, as a result of growing demand for personalized medicines, rising investment in R&D, and adoption of decentralized clinical trials.
The single-use bioprocessing market is a rapidly growing industry of biopharmaceutical industry. Single-use bioprocessing is a method of manufacturing biopharmaceuticals that uses disposable, single-use equipment instead of traditional stainless-steel equipment. This method has several advantages, including reduced contamination risk, decreased setup time, and improved flexibility.
The market growth is driven by several factors, including the increasing demand for biologics the growing biopharmaceutical industry, and the advantages of single-use bioprocessing over traditional manufacturing methods. The market is highly competitive, with several key players investing heavily in research and development activities to stay ahead of the competition.
Biologics are complex molecules that are manufactured using living cells and are used to treat a varied range of diseases, including infectious diseases, cancer, and autoimmune disorders. The increasing demand for biologics is driving the growth of the single-use bioprocessing industry, as single-use bioprocessing is particularly well-suited for the production of these complex molecules. Geographically, North America is expected to dominate the single-use bioprocessing sector during the forecast period, followed by Europe and Asia Pacific. This can be attributed to the presence of a large number of biopharmaceutical companies in these regions, as well as favorable government regulations and initiatives.
However, the market of single-use bioprocessing also faces some challenges, such as concerns about the quality and consistency of single-use products, the lack of standardization in the industry, and the high cost of single-use equipment. These challenges are expected to be addressed through technological advancements, standardization efforts, and increased competition in the market.
Single-Use Systems (SUS) offer accelerate processing times, reduce operational costs, and enable faster market entry by shortening time-to-market. SUS provides flexibility to adapt production capacities according to market demands and supports the manufacturing of multiple products in a single facility, minimizing cross-contamination risks.
Additionally, SUS simplifies contamination control, leading to fewer quality and regulatory concerns, which enhances productivity and speeds up the bioprocessing of vaccines. These systems also lower facility costs, require less validation, and allow for quicker changeovers. Currently, SUS is utilized across various bioprocessing stages, from upstream expression to fill-finish, and is often more cost-effective compared to stainless steel systems.
The single-use bioprocessing industry is rapidly evolving, and automation is expected to play a significant role in its future development. Automation offers numerous benefits to the biopharmaceutical manufacturing process, including increased efficiency, improved product quality, and reduced labor costs.
One of the significant rewards of automation in single-use bioprocessing is its ability to improve process control. Automation can provide real-time monitoring of critical process parameters, enabling manufacturers to detect deviations and make adjustments in real-time. This results in better product quality and consistency, as well as reduced process variability. Another advantage of automation in single-use bioprocessing is its ability to increase efficiency. Automation can reduce the need for manual intervention, leading to faster and more consistent processes. This can result in increased throughput and reduced production time, enabling manufacturers to bring products to market more quickly.
In addition, automation can also reduce labor costs, as it eliminates the need for manual labor in many areas of the manufacturing process. This can result in significant cost savings, particularly in high-labor areas such as cell culture and downstream processing. Furthermore, automation in single-use bioprocessing can also improve worker safety. Many tasks in biopharmaceutical manufacturing involve working with hazardous materials, and automation can reduce the risk of exposure to these materials. This can improve worker safety and reduce the likelihood of accidents and injuries. The benefits of automation in single-use bioprocessing are significant, and many manufacturers are already implementing automated systems in their facilities.
Thus, automation is expected to dominate the single-use bioprocessing industry within the next five years. Automation offers numerous benefits, including improved process control, increased efficiency, reduced labor costs, and improved worker safety. As manufacturers continue to seek ways to improve efficiency and reduce costs, automation is likely to become an increasingly important part of the biopharmaceutical manufacturing process.
The energy consumption of stainless-steel systems and single-use systems can vary significantly. Stainless steel systems require more energy to clean and sterilize between batches, as well as to maintain temperature control throughout the production process. The high energy demand associated with stainless steel systems is due to the need for steam and hot water, which are required for cleaning and sterilization.
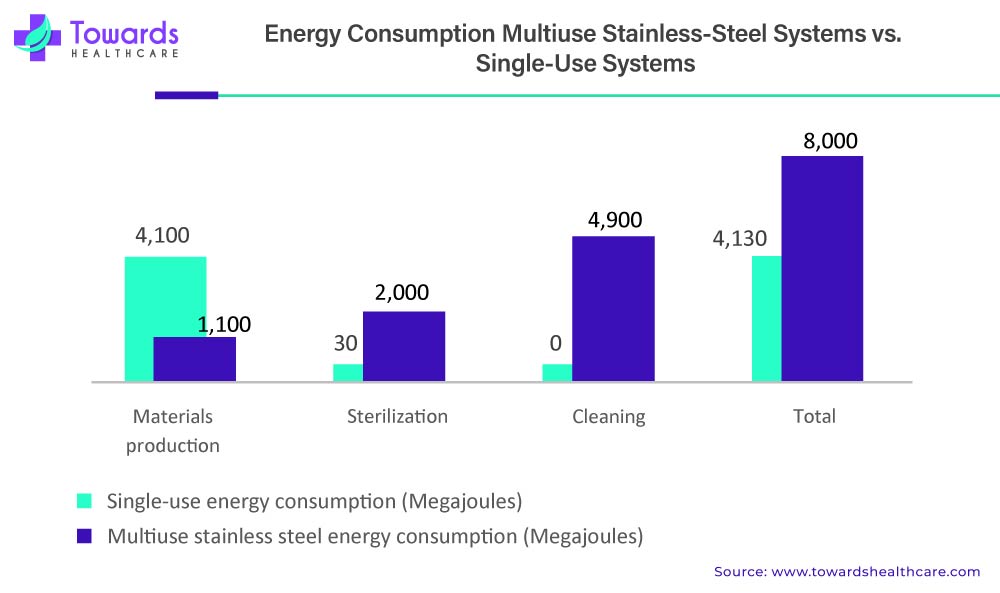
Single-use systems, on the other hand, require less energy than stainless steel systems because they do not require cleaning and sterilization between batches. Single-use systems are designed for single-use, eliminating the need for cleaning and sterilization, which significantly reduces energy consumption. Furthermore, the materials used in single-use systems are lightweight, which can also reduce energy consumption during transportation and handling.
Another factor that contributes to the energy consumption of bioprocessing systems is the cooling and heating of media. Single-use systems can also have advantages in this aspect, as they are designed to have a higher surface area-to-volume ratio, which can improve heat transfer rates and reduce energy consumption.
The US FDA has approved more than 20 gene therapies and around 1000 gene therapy molecules are in the stages of clinical trials, reported as of February 2023.
Cell and gene therapies are emerging as promising treatments for a wide range of diseases, including cancer, genetic disorders, and autoimmune diseases. These therapies involve the use of living cells or genetic material to treat or cure diseases, and they require complex manufacturing processes. Single-use technology has emerged as a game-changing solution for the manufacture of cell and gene therapies, offering a range of benefits over traditional stainless-steel equipment.
The bioprocessing industry has been using single-use technology for several years, and the lessons learned from this industry can be applied to the manufacture of cell and gene therapies. The key benefits of single-use technology in the bioprocessing industry include reduced contamination risk, increased flexibility, and reduced turnaround time. These benefits also apply to the manufacture of cell and gene therapies.
One of the key advantages of single-use technology in the manufacture of cell and gene therapies is the ability to eliminate the need for cleaning and sterilization between batches. This reduces the risk of contamination and cross-contamination, which is particularly important in the manufacture of cell and gene therapies, where the cells or genetic material being used are extremely sensitive to contamination.
Cell and gene therapies are often developed for small patient populations, which means that manufacturing processes must be adaptable to changing needs. Single-use technology allows for quick and easy changes to manufacturing processes, allowing manufacturers to produce small batches of products without the need for significant reconfiguration of equipment.
In addition, single-use technology can significantly reduce the turnaround time for manufacturing processes. This is because single-use systems can be pre-sterilized and pre-assembled, reducing the time required for cleaning and preparation between batches. This results in faster production times, which is critical for the timely delivery of cell and gene therapies to patients.
Furthermore, single-use technology can also help reduce the capital costs associated with the manufacturing of cell and gene therapies. Traditional stainless-steel equipment can be expensive to install and maintain, whereas single-use systems can be more cost-effective and require less physical space. This can be particularly beneficial for small and mid-sized manufacturers who may have limited resources.
Biologics are complex molecules that are manufactured using living cells and are used to treat an extensive range of diseases, including cancer, autoimmune disorders, and infectious diseases. The demand for biologics is increasing rapidly, driven by several factors such as the aging population, increasing prevalence of chronic diseases, and technological advancements in biopharmaceutical manufacturing. To meet the growing demand for biologics, the biopharmaceutical industry is increasingly turning to single-use bioprocessing technology. Single-use bioprocessing technology offers several advantages over traditional stainless-steel equipment, including increased flexibility, reduced contamination risk, and shorter turnaround times.
Single-use bioprocessing technology is particularly well-suited for the production of biologics because it allows manufacturers to quickly and easily switch between different products or production processes. This flexibility is crucial in the biologics industry, where production runs can be short and manufacturers need to be able to quickly adapt to changing market demands. Single-use bioprocessing technology also reduces the risk of contamination and cross-contamination, which is particularly important in the manufacture of biologics. Biologics are complex molecules that are sensitive to even minor changes in the manufacturing process, and any contamination can have a significant impact on the safety and efficacy of the final product.
In addition, single-use bioprocessing technology offers shorter turnaround times than traditional manufacturing methods. Biologics are often used to treat life-threatening conditions, and any delays in manufacturing can have serious consequences for patients. Single-use bioprocessing technology allows manufacturers to quickly set up and tear down production runs, reducing the time required to manufacture the therapies. Beyond biologics, single-use bioprocessing technology is also being used in the manufacture of other types of products, including vaccines, cell and gene therapies, and biosimilars. The advantages of single-use bioprocessing technology, including increased flexibility and reduced contamination risk, make it an attractive option for the manufacture of these complex products.
The single-use bioprocessing market is expected to grow significantly in the coming years, driven by several prominent growth drivers. One of the key growth drivers for the single-use bioprocessing market is the increasing demand for biologics. Single-use bioprocessing is particularly well-suited for the production of biologics because it offers several advantages over traditional manufacturing methods. For example, single-use technology eliminates the need for cleaning and sterilization between batches, reducing the risk of contamination and cross-contamination. It also offers increased flexibility, allowing manufacturers to quickly and easily switch between different products or production processes. Single-use bioprocessing technology offers several advantages over traditional manufacturing methods, including increased flexibility, reduced contamination risk, and shorter turnaround times. As the biopharmaceutical industry continues to grow, single-use bioprocessing technology is expected to play an increasingly important role in the manufacture of biologics and other complex products.
The surge in demand for antibody-drug conjugates (ADCs) in the biopharmaceutical drug market has highlighted the need for specialized manufacturing processes and facilities. ADCs merge the potency of small molecules and the targeting capability of monoclonal antibodies, which requires extreme control measures during manufacturing. This has led to the recommendation of single-use containment systems for ADC handling.
Single-use technology can offer the required controls while minimizing manufacturing costs and enabling the operation of multi-use facilities. Flexible single-use isolators have been proven to meet the high-containment performance required for handling ADCs by risk assessment studies. Isolators can be used to manage solids in the chemical synthesis of harmful materials and downstream processing when managing liquids to protect from aerosolization and spills.
In addition to the cost benefits, single-use technology can also improve the efficiency and speed of ADC manufacturing. The elimination of cleaning and sterilization steps between batches reduces downtime and turnaround time, enabling faster production and greater capacity for meeting the demand for ADCs.
There are over 50 biopharma enterprises developing ADCs to treat cancer as well as a range of tumors, including lung, bladder, and gynecological tumors. Antibody–drug conjugates (ADCs) are growing in popularity, with a healthy pipeline that could lead to a market worth billions of dollars over the next few years. These novel treatments merge the high potency of small molecules and the targeting capability of monoclonal antibodies (MAbs). However, the question of such treatments is whether they merge a toxic, highly potent material with a large protein that will aim for a treatment area. As a result, small molecule manufacturing requires extreme controls which is specific to facility design, personal protective equipment (PPE), high-containment isolators, and operator training.
Single-use containment systems are recommended for ADC handling. Such systems can offer the required controls while minimizing the manufacturing cost to enable the operation of multi-use facilities. Further, single-use flexible isolators have been proven to meet the high-containment performance required for handling ADCs (<30.0 ng/m3) by risk assessment studies. Isolators can be used both for managing solids in the chemical synthesis of harmful materials and in downstream processing when managing liquids to protect from aerosolization and spills.
The use of fully disposable technology in biopharmaceutical manufacturing, including single-use bioprocessing, has been steadily increasing over the past several years. However, the adoption of fully disposable technology on a widespread basis is still in the early stages. There are several factors that are driving the shift towards fully disposable technology in biopharmaceutical manufacturing. One of the key drivers is the increasing demand for flexible manufacturing solutions. Fully disposable technology allows manufacturers to quickly and easily switch between different products or production processes, which is crucial in the biopharmaceutical industry, where production runs can be short and manufacturers need to be able to quickly adapt to changing market demands. Another driver is the increasing emphasis on contamination control. Fully disposable technology can reduce the risk of contamination and cross-contamination, which is particularly important in the manufacture of biologics and other complex products. Biologics are sensitive to even minor changes in the manufacturing process, and any contamination can have a significant impact on the safety and efficacy of the final product.
In addition, fully disposable technology can also reduce the need for cleaning and validation, which can save time and reduce costs. Traditional stainless-steel equipment requires extensive cleaning and validation between production runs, which can be time-consuming and expensive. Fully disposable technology eliminates the need for cleaning and validation, allowing manufacturers to reduce downtime and increase throughput.
Despite the advantages of fully disposable technology, the transition to 100% fully disposable facilities is likely to be a gradual process. There are still some challenges to be overcome, such as the need for increased supply chain security and the development of standardized interfaces between equipment. In addition, fully disposable technology may not be suitable for all applications, particularly those that require large-scale production. However, many manufacturers are already using fully disposable technology for certain applications, and the trend toward increased adoption is expected to continue.
By product, the simple & peripheral elements segment held a dominant presence in the single-use bioprocessing market in 2024. Some common components of simple and peripheral elements include tubing, filters, connectors, and sensors. These elements are used to connect and facilitate the flow of materials within a single-use bioprocessing system. These elements are widely used to reduce the risk of contamination and decrease the need for complex cleaning procedures. They are affordable and lead to increased productivity. These components help maintain the physical integrity of a bioprocess setup. Regular maintenance and technical defects increase the chance of using these elements.
By product, the apparatus & plants segment is expected to grow at the fastest rate in the market during the forecast period. Apparatus and plants are essential components of a single-use bioprocessing system. They include bioreactors, mixing, storage, & filling systems, filtration, and chromatography systems. These components carry out all the processing required for manufacturing biologicals and other products. The increasing investments and favorable regulatory frameworks to set up a manufacturing facility potentiate the demand for apparatus & plants during single-use bioprocessing.
By workflow, the upstream bioprocessing segment held the largest share of the global single-use bioprocessing market in 2024. Upstream bioprocessing refers to a process in which microbes/cells are grown from bacterial or mammalian cell lines. These small quantities of cell culture are then utilized to produce larger quantities in a controlled environment of bioreactors. The increasing demand for large-scale manufacturing of biopharmaceuticals owing to rising incidences of diseases and new product launches propel the segment’s growth.
By workflow, the fermentation segment is predicted to witness significant growth in the market over the forecast period. Fermentation is an essential process in single-use bioprocessing systems. It is used for manufacturing a wide range of pharmaceutical products from recombinant proteins to vaccines and antibiotics. The growing demand for biopharmaceuticals supports the segment’s growth. The rising demand for small biologicals and increased yields for biopharmaceutical production promote the use of the fermentation method. Additionally, rapid advancements in molecular biology and synthetic biology contribute to the segment’s growth.
By end-use, the biopharmaceutical manufacturers segment led the global single-use bioprocessing market in 2024. The presence of favorable infrastructure and suitable capital investment drive the segment’s growth. Single-use bioprocessing systems are predominantly used for manufacturing biopharmaceuticals. The growing demand for biopharmaceuticals and new product launches necessitate large-scale manufacturing of biopharmaceuticals. Favorable regulatory frameworks regulate the use of single-use bioprocessing systems. The increasing number of CROs and CMOs for manufacturing enables scalability and flexibility.
By end-use, the academic & clinical research institutes segment is projected to expand rapidly in the market in the coming years. Several government and private organizations provide funding for manufacturing requirements. The growing research and development activities in academic and research institutes lead to the development of novel products. Some institutes offer contract services for research based on biopharmaceuticals and microbiology. Academic and research institutes organize workshops and symposiums to train individuals in operating bioprocessing systems.
North America dominated the global single-use bioprocessing market in 2024. The presence of key players and favorable government support drive the market. The U.S. government announced an investment of $2 billion for manufacturing biologicals under the National Biotechnology and Biomanufacturing Initiative in 2023. This leads to enhanced adoption of single-use bioprocessing systems. The U.S. and Canadian governments also impose stringent guidelines related to environmental sustainability, potentiating the demand for single-use systems. Suitable regulatory frameworks and new product launches also contribute to the market. The U.S. Food and Drug Administration approved a total of 50 novel drugs, out of which 18 were new biological entities.
Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period. The rising incidences of chronic disorders and increasing investments drive the market. Countries like China, India, and Japan are at the forefront of driving the market growth in Asia-Pacific. The availability of suitable manufacturing infrastructure also promotes market growth. This enables numerous foreign investors and manufacturers to set up a manufacturing facility at affordable prices. Favorable government policies such as “Made in China” and “Make in India” encourage indigenous manufacturing of biopharmaceuticals. Additionally, the burgeoning pharmaceutical and biotechnology sector, due to the increasing number of biotech companies and increasing investment, augments the market.
Europe is expected to experience significant growth in the single-use bioprocessing market during the forecast period. Europe is experiencing growth due to technological advancements in the industries. At the same time, the rising incidence of the diseases is increasing the demand for new diagnostic and treatment options. Similarly, the use of biologics as well as personalized medications is also increasing. Thus, these new developments are leading to a rise in the use of single-use bioprocessing systems. This, in turn, promotes the market growth.
Germany Market Trends
The industries in Germany are developing as they are utilizing technologically advanced instruments for improving the production process. Moreover, the use of personalized treatments is also rising due to their effectiveness as well as growing awareness. Thus, the use of single-use bioprocessing systems also increases. This helps in amplifying the production as well as maintaining the quality of the products developed.
UK Market Trends
The collaboration in the UK is rising for the development of new treatment options, as well as the adoption of single-use bioprocessing systems, is also increasing. This, in turn, helps to develop personalized medications as well as biologics for various disease treatments. It is further supported by the government as well as the regulatory bodies.
Michael May, President and CEO of CCRM, commented that the future of single-use bioprocessing involves addressing the high cost of manufacturing cell and gene therapies, leading to transformative treatment afforded and adopted by health systems. He also said that the company’s collaboration with Membio drives efficiency in manufacturing processes and increases the accessibility of life-saving medicines.

By Product
By Workflow
By End-Use
By Region
February 2026
February 2026
February 2026
February 2026