November 2025

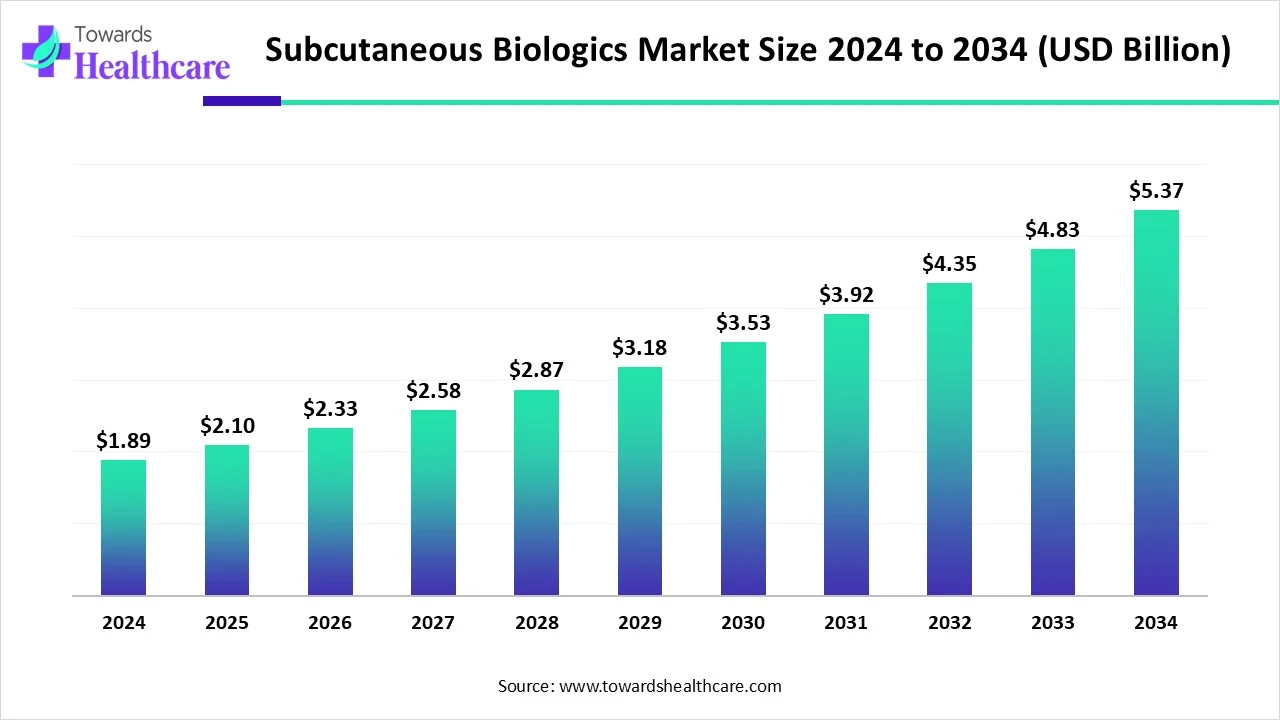
The global subcutaneous biologics market size is calculated at USD 1.89 billion in 2024, grew to USD 2.1 billion in 2025, and is projected to reach around USD 5.37 billion by 2034. The market is expanding at a CAGR of 11.09% between 2025 and 2034.
The subcutaneous biologics market growth is attributed to the increasing demand for biologics as therapeutics. The rising need for home healthcare also drives market growth, enhancing patient convenience and medication adherence. Several government organizations provide funding to support research activities for developing novel biologics and drug delivery systems.
Advanced and innovative drug delivery systems for painless administration of biologics present future opportunities for the market. Additionally, the integration of AI and ML in subcutaneous drug delivery systems can revolutionize the market.
| Metric | Details |
| Market Size in 2025 | USD 2.1 Billion |
| Projected Market Size in 2034 | USD 5.37 Billion |
| CAGR (2025 - 2034) | 11.09% |
| Leading Region | North America |
| Market Segmentation | By Biologic Type, By Administration Method, By Therapeutic Area, By Region |
| Top Key Players | AbbVie, Inc., Biocon Biologics Ltd., Biogen, Inc., Eisai Co. Ltd., F. Hoffman-La Roche, Genentech, Inc., Lindy Biosciences, Merck, Novartis Pharma AG, Pfizer, SCHOTT Pharma, Takeda Pharmaceuticals |
Biologics are advanced therapies derived from living organisms such as cell and gene therapy, immunotherapy, vaccines, blood and blood components, allergenics, and monoclonal antibodies. These biologics are either administered via intravenous (i.v.) or subcutaneous (s.c.) route within the human body. Subcutaneous administration refers to injecting biologics directly into the subcutaneous tissue. Tissue, enabling slower absorption into the central circulation via blood capillaries or indirectly via lymphatics. Subcutaneous administration is widely preferred as it has been proven effective, safe, and well-tolerated. It results in reduced drug delivery-related healthcare costs and resource use.
Numerous factors influence market growth, including the growing demand for biologics as therapeutics and the rising need for self-administration. The growing research and development activities lead to the development of novel biologics and drug delivery systems. The creation of fixed-dose combinations and ready-to-use devices is a result of technological advancements in the field. The burgeoning biotech sector and increasing collaborations contribute to market growth. Moreover, changing patient preferences and the need for safe administration promote the demand for subcutaneous biologics.
Artificial intelligence (AI) plays a vital role in automating the delivery of complex biologics. Integrating AI into drug delivery systems can measure the amount and time of dose delivery. It can also send reminders to patients about the delivery time and improve personalized dosing regimens. AI can also provide real-time data about patients’ drug administration to healthcare professionals. This enables healthcare professionals to get real-time insights into patients’ medication adherence. Moreover, AI can ensure that drug delivery systems meet regulatory standards by analyzing data for compliance and identifying potential issues.
Self-Administration
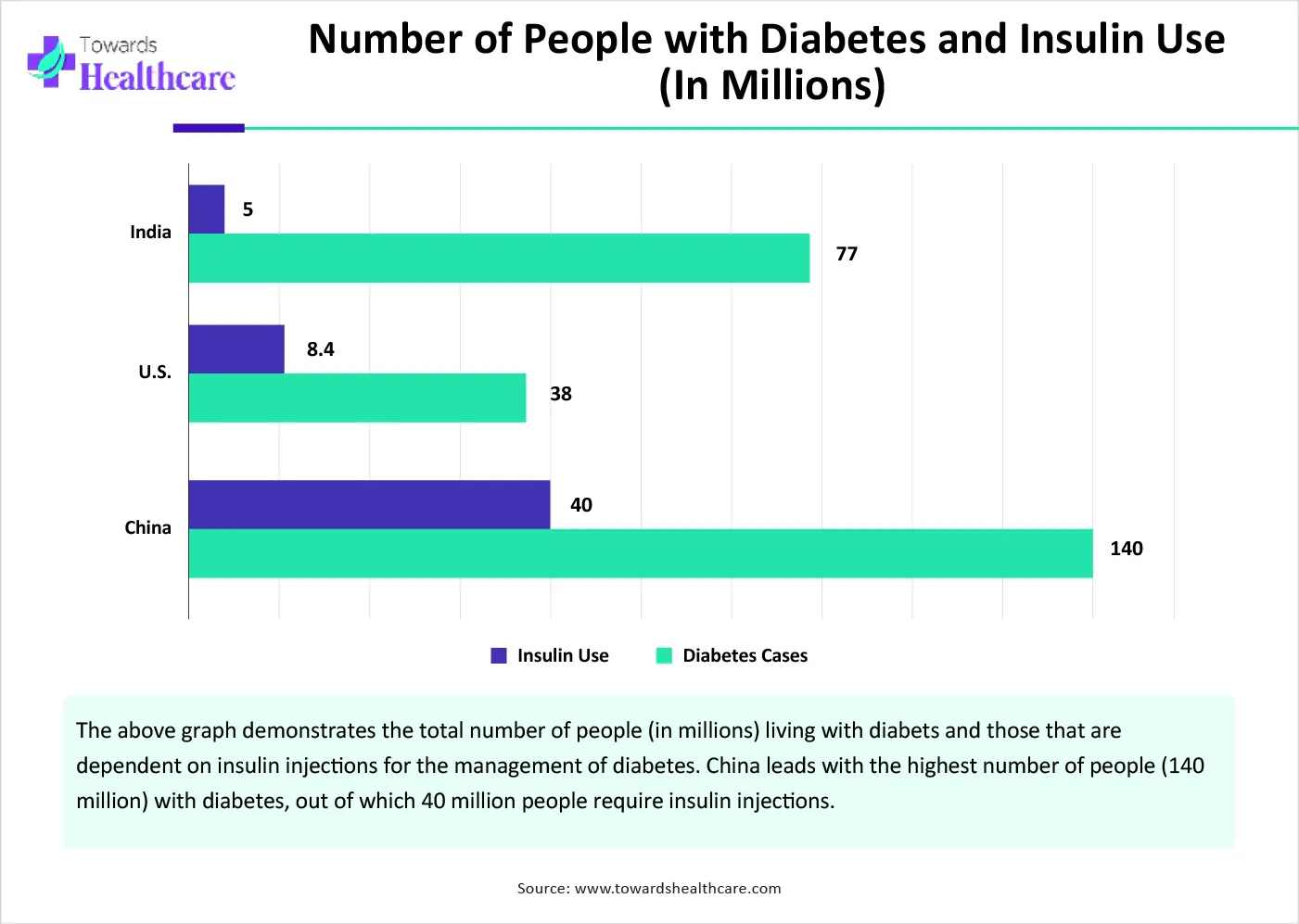
The major growth factor of the subcutaneous biologics market is the increasing preference for self-administration. Self-administration eliminates the need for skilled professionals to deliver injectables. It increases independence, improves medication knowledge, and enhances patient empowerment. It also promotes patient-centered care and reduces the need for frequent hospital visits, enhancing patient medication adherence. Subcutaneous biologics are most widely used by patients with type 1 or type 2 diabetes for insulin administration. Thus, the rising prevalence of type 1 or type 2 diabetes is likely to potentiate the need for s.c. Biologics.
Technical Issues
The major issues related to subcutaneous injections are the administration of only low-volume biologics and a slower rate of absorption. Subcutaneous injections can only deliver medications up to 1-2 mL, posing challenges in drug delivery. Slow rate of absorption also leads to slower onset of action, restricting market growth.
What is the Future of the Subcutaneous Biologics Market?
The future of the market is promising, driven by advancements in drug delivery technologies. Ongoing efforts are made to develop drug delivery devices that are convenient for the precise administration of biologics. The increasing adoption of the needle-free jet injection device reduces needle-stick infections and eliminates the need for painful administration. Some drug delivery systems administer a precise dose of biologics with one click. Novel systems are being developed to improve the stability and duration of biologics, including polymeric micronanocarriers, in-situ hydrogels, liposomes, and inorganic nanoparticles. The preparation approach is improved to reduce the burst release of peptides or biologics and increase their entrapment efficiency.
By biologic type, the antibodies segment held a dominant presence in the market in 2024. Antibody-based therapeutics are widely preferred due to their ability to bind specifically to an antigen and provide targeted action. They have comparatively fewer adverse effects compared to other biologics due to their high specificity. Additionally, they can activate the immune system, leading to long-lasting immunity to specific diseases, such as infectious disorders. The U.S. Food and Drug Administration (FDA) approved a total of 12 antibody-based therapeutics in 2023. (Source - Oxford Academic). Advancements in protein engineering technology lead to the development of bispecific antibodies that can bind to multiple antigens simultaneously.
By biologic type, the nucleotides segment is expected to grow at the fastest CAGR in the market during the forecast period. The demand for nucleotide-based or nucleic acid-based therapeutics is increasing as it can offer huge potential to modulate cellular pathways. These therapeutics affect gene expression within cells and change protein expression, altering the course of a disease. Thus, they can treat a disease from its root cause. The growing genomics research and advancements in genetic engineering technology boost the segment’s growth. The different types of nucleotide-based therapeutics are antisense oligonucleotides (ASOs), small interfering RNA (siRNA), messenger RNA (mRNA), and self-amplifying RNA (saRNA).
By administration method, the injection segment dominated the global market in 2024. This segment dominated due to the ease of administration and faster administration. Injection is given in a ‘shot’ using a needle or syringe and usually takes a short period, unlike infusion. It is administered in the fat layer, beneath the skin. It does not need specific equipment for administering biologics, making it convenient for both patients and healthcare professionals. Some examples of biologics that are administered via injection include insulin, certain hormone medications like testosterone, blood thinners, allergy medications, analgesics, and arthritis medications.
By administration method, the infusion segment is expected to witness significant growth in the market over the forecast period. Infusion refers to delivering a medication for a longer duration, either continuously or in spurts. Biologics are administered using infusion pumps that can be time-controlled. Subcutaneous infusions are absorbed in the body more slowly than other types of infusion therapy, but faster than oral therapy. They are widely used for administering fertility drugs, medications for arthritis and autoimmune disorders, and opioids.
By therapeutic area, the autoimmune disorders segment led the global market in 2024. The segmental growth is attributed to the rising prevalence of autoimmune disorders and the need for personalized medicines. The global prevalence rate of psoriasis, an autoimmune disorder, is estimated to be 2-3% of the total population. Biologics are prominent therapeutic alternatives for autoimmune disorders as they aid in targeted therapy and reduce systemic side effects. Conventional therapeutics fail to cure a disease from its root cause, thereby potentiating the demand for subcutaneous biologics.
By therapeutic area, the oncological disorders segment is expected to grow with the highest CAGR in the market during the studied years. The rising prevalence of cancer and its complexity augment the segment’s growth. The World Health Organization (WHO) predicted that over 35 million new cancer cases will be reported by 2050. (Source - WHO) The growing research and development activities lead to new product launches. Advances in genomics enable researchers to study the disease progression and develop novel biologics based on patients’ conditions.
North America held the largest revenue share of the market in 2024. The major growth factors that govern market growth in North America are the presence of key players, the growing demand for biologics, and the availability of a robust healthcare infrastructure. State-of-the-art research and development facilities and increasing funding by government and private organizations facilitate the development of novel biologics. The rising adoption of advanced technologies also contributes to market growth.
Key players, such as Pfizer, Genentech, Inc., and Biogen, Inc., are the major contributors to the market in the U.S. The FDA approved about 18 biologics, out of the 50 new molecular entities approved in 2024. The “Precision Medicine Initiative” was launched to advance precision medicine in research centers in the U.S.
Health Canada works to maximize the safety and effectiveness of biologics, including vaccines and biotechnology products, as well as radiopharmaceuticals in the Canadian marketplace and health system. There are around 23 monoclonal antibodies, both biologic and biosimilar, available in Canada.
Asia-Pacific is expected to host the fastest-growing subcutaneous biologics market in the coming years. The demand for personalized medicines is increasing due to rapidly changing demographics and the growing patient population. The burgeoning biotech sector and the increasing number of biotech startups contribute to market growth. The rising prevalence of chronic disorders and the geriatric population potentiate the need for biologics. Favorable government support plays a crucial role in fostering market growth.
The National Medical Products Administration (NMPA) regulates the approval of biologics in China. In 2024, the NMPA approved a total of 228 new drug applications, of which 93 were biologics. Out of 93, 18 were innovative biologics and 24 were modified. (Source - Nature) The NMPA also launched a pilot plan to allow non-end-to-end manufacturing of biologics.
India has the world’s highest number of approved biosimilars, accounting for 95. The increasing number of clinical trials also potentiates market growth. As of June 11, 2025, about 54 clinical trials were registered on the clinicaltrials.gov website related to biologics as an intervention in India. (Source - Clinical Trials) Approximately 53% of all deaths in India are due to non-communicable diseases or chronic disorders.
Europe is expected to grow at a considerable CAGR in the subcutaneous biologics market in the upcoming period. Numerous government organizations support the development of biologics through various initiatives and funding. They also launch initiatives to promote screening and early diagnosis of chronic disorders, enabling healthcare professionals to provide personalized treatment. The growing research and development activities and favorable healthcare infrastructure encourage healthcare organizations to adopt innovative technologies and products.
The German government formed a national pharma strategy to support companies, streamlining approval procedures and improving conditions for R&D in Germany. This encourages foreign countries to set up their manufacturing facility in Germany. In August 2024, Sanofi, a French pharmaceutical giant, announced an investment of 1.3 billion euros to expand insulin production in the western German city of Frankfurt am Main. (Source - GTAI)
The UK government is also at the forefront of supporting biologics research and manufacturing in the nation. The government announced an investment of £277 million as part of the “Life Sciences Innovative Manufacturing Fund” (LSIMF) grants to help fund and advance life sciences manufacturing projects in both medical diagnostics and human medicines. (Source - Gov.uk)

Dr. Enriqueta Felip, Head of Thoracic Tumors Group, Vall d’Hebron Institute of Oncology, commented that the study findings demonstrated subcutaneous pembrolizumab, developed by Merck, reduces time demands for both the patient and the healthcare providers, all while providing a consistent efficacy and safety profile with IV pembrolizumab. She also said that if subcutaneous pembrolizumab is approved, it can give patients valuable time back in their treatment day, with results consistent with those of IV pembrolizumab. (Source - Businesswire)
By Biologic Type
By Administration Method
By Therapeutic Area
By Region
November 2025
November 2025
November 2025
November 2025